- Dystonia: Causes & Treatment
- Zero Stroke: Meaning & Causes
- Subnormal, Supernormal and Paranormal in Abnormal Psychology
- Meaning of 4 D’s in Abnormal Psychology
- International Classification of Diseases
- Insanity Defense: Meaning & Applicability
- Wernicke-Korsakoff Syndrome
- Vertigo: Symptoms, Causes, and Treatments
- Transvestic Disorder
- Quadriplegia
- Myotonia Congenita
- Lafora Disease
- Prosopagnosia
- Microcephaly
- Macrocephaly
- Lissencephaly
- Isodicentric 15
- Dysautonomia
- Double Vision
- Developmental Coordination Disorder
- Dawson Disease
- Dandy-Walker Syndrome
- Palinopsia
- Angelman Syndrome
- Alien Hand Syndrome
- Sexual Sadism Disorder
- Sexual Masochism Disorder
- Parkinson’s Disease
- Cyclic Vomiting Syndrome
- Craniosynostosis
- Confusional Arousals
- Rapid Eye Movement Sleep Behavior Disorder
- Corticobasal Degeneration
- Coma: Symptoms & Causes
- Color Blindness
- Cognitive Model of Abnormal Psychology
- Zellweger Syndrome
- Hypoxia
- Hypersomnia: Meaning & Symptoms
- Coffin-Lowry Syndrome
- Cockayne Syndrome
- Cluster Headache
- West Syndrome
- Voyeuristic Disorder
- Sleep-Related Eating Disorders
- Sleep Related Movement Disorders
- Pyromania
- Psychotic Major Depression
- Psychodynamic Model of Abnormal Psychology
- Pedophilia: A Disorder of Sexual Attraction For Minors
- Olfactory Reference Syndrome
- Neuroticism: Meaning and Effect
- Neurogenetic Disorders
- Narcolepsy: A Sleep Disorder
- Kleptomania: Symptoms & Causes
- Intermittent Explosive Disorder
- Insomnia: A Sleep Disorder
- Hypoesthesia
- Fragile X Syndrome
- Fibromyalgia
- Febrile Seizures
- Fahr’s Syndrome
- Extraversion Vs. Introversion: Hans Eysenck
- Encephalitis
- Dyskinesia
- Dysgraphia
- Cocaine Induced Anxiety
- Chronic Fatigue Syndrome
- Chorea
- Cerebral Arteriosclerosis
- Cerebral Aneurysm
- Cephalic Disorder
- Centronuclear Myopathy
- Central Pontine Myelinolysis
- Central Pain Syndrome
- Causalgia
- Carpal Tunnel Syndrome
- Canavan Disease
- Brody Myopathy
- Brain Death
- Attention Deficit Hyperactivity Disorder
- ATR-16 Syndrome
- Ataxia
- Asomatognosia
- Arachnoiditis
- Apraxia: A Neurological Disorder
- Aphasia: Spoken Language Disorder
- Akinetopsia: A Disorder of Motion Blindness
- Aicardi Syndrome
- Agraphia: Lost of Writing Skill
- Agnosia: A Disorder of Memory Loss
- Achromatopsia: A Disorder of Color Vision
- Abulia: A Neurological Disorder
- Genito-pelvic Pain/penetration Disorder
- Frotteurism Disorder
- Exhibitionistic Disorder
- Avoidant/Restrictive Food Intake Disorder
- FIRO-B
- Aphantasia
- Anosognosia: A Disorder of Loss of Insight
- Oppositional Defiant Disorder
- Kleine- Levin Syndrome
- Internet Gaming Disorder
- Inhalant-Related Psychiatric Disorders
- Encopresis (Involuntary Defecation)
- Atypical Depression
- Synthetic cathinone-induced Psychotic Disorder
- Reactive Attachment Disorder
- Insufficient Sleep Syndrome
- Disinhibited Social Engagement Disorder
- Corticobasal Syndrome
- Amnesia: A Mental Disorder of Forgetting Things
- Traumatic Brain Injury
- Trance and Possession Disorder
- Sedative, Hypnotic, or Anxiolytic Induced Psychotic Disorder
- Premature Ejaculation
- Enuresis (Involuntary Urination)
- Exploding Head Syndrome
- Common Defense Mechanisms Used for Anxiety
- Caffeine-Induced Anxiety Disorder
- Benzodiazepine-Induced Mood Disorders
- Alcohol-Induced Mood Disorders
- Non-24-Hour Sleep-Wake Disorder
- Mild Neurocognitive Disorder
- Jet Lag
- Cocaine-Induced Psychosis
- Amphetamine-Induced Psychosis
- Cannabis-Induced Psychosis
- Psychological Disorders: Meaning and Types
- Prolonged Grief Disorder
- Partial Dissociative Identity Disorder
- Models of Abnormal Psychology
- Mental Health Vs. Mental Illness
- Dissociative Amnesia with Dissociative Fugue
- Behavior Model of Abnormal Psychology
- Biological Model of Abnormal Psychology
- Schizotypal Personality Disorder
- Stigma Around Substance Abuse Disorder
- Schizophreniform Disorder
- Schizoid Personality Disorder
- Obsessive-Compulsive Personality Disorder
- Coping with Stress
- Avoidant Personality Disorder
- Antisocial Personality Disorder
- Histrionic Personality Disorder
- Drug Use Disorders
- Dependent Personality Disorder
- Borderline Personality Disorder
- Paranoid Personality Disorder
- Nightmare Disorder
- Personality Stability and Change
- Paraphilic Disorders
- Gender Dysphoria
- Pica: Causes, Symptoms, & Treatment
- Motor Disorder
- Gambling Disorder
- Female Orgasmic Disorder
- Erectile Disorder
- Disruptive Impulse Control and Conduct Disorders
- Dementia: Symptoms and Causes
- Delirium: Meaning & Symptoms
- Conduct Disorder
- Anxiety: A Threat to the Ego
- Alzheimer’s Dementia
- Alcohol Use Disorder
- Vascular Dementia
- Narcissistic Personality Disorder
- Lewy Body Dementia
- Huntington’s Disease
- ChildhoodOnset Fluency Disorder (Stuttering)
- Speech Sound Disorder (Phonological Disorder)
- Sleepwake Disorders
- NonRapid Eye Movement Sleep Arousal Disorders
- Elimination Disorders
- Attention Deficit/Hyperactivity Disorder: A Spinning Top
- Gender Differences in Schizophrenia
- Neurodevelopmental Risk Factors in Schizophrenia
- Developmental Processes in Schizophrenic Disorders
- Schizophrenia Symptoms: Positive and Negative?
- Schizophrenia: Meaning, Symptoms & Treatment
- Suicide: Definition and Meaning
- Seasonal Affective Disorder (S.A.D.)
- Learning Disorders
- Language Disorders
- Catatonia: Meaning & Treatment
- Persistent Delusional Disorder
- Male Hypoactive Sexual Desire Disorder
- Brief Psychotic Disorder (BPD)
- Female Sexual Interest/Arousal Disorder (FSIAD)
- Schizoaffective Disorder
- Rumination Disorder
- Grief and Trauma Counselling
- Fetal Alcohol Syndrome
- Restless Legs Syndrome
- Various Types of Mood Disorders
- Bulimia Nervosa
- Bipolar II Disorder
- Bipolar I Disorder
- Binge Eating Disorder
- Separation Anxiety Disorder (SAD)
Personality Psychology
- Kretschmer’s Classification
- Metamotivation: Meaning and Significance
- Three Essays on The Theory of Sexuality
- The Psychopathology of Everyday Life
- Criticisms of Carl Roger’s Theory
- Aspects of Personality: Carl Jung
- Feminist Perspective on Erikson’s Theory
- Gender Identity and Sexual Orientation in Erickson’s Theory
- Self-Actualization: Meaning & Significance
- Rogers’ Humanist Theory of Personality
- Raymond Cattell’s Theory of Personality
- Personality Development in Childhood: The Unique Self
- Gordon Allport: As a Psychologist
- The Decline and Fall of the Freudian Empire
- Television Violence and Aggressive Behaviour: Albert Bandura
- Anticipating Life Events: George Kelly
- Person-Centered Therapy: Carl Rogers
- Biological Theory of Intelligence: Hans Eysenck
- Generativity vs. Stagnation: Erik Erikson
- Emotional Adjustment: Meaning and Significance
- Eysenck’s Pen Model of Personality
- Criticism of Allport’s Trait Theory of Personality
- Criticism of Freud’s Psychoanalysis
- Harmony Between Personalities
- Carl Rogers: As a Psychologist
- Albert Bandura: As a Psychologist
- Trust vs Mistrust: Erik Erikson
- Reinforcement Theory of Motivation – B. F. Skinner
- Reinforcement and Punishment: B.F. Skinner
- Oedipus Complex and Electra Complex
- Neuroticism vs. Emotional Stability
- Mother -Infant Bonding: Allport’s Theory
- Eysenck’s Personality Theory
- Cultural Differences in Facial Expression: Allport’s Theory
- Initiative vs. Guilt: Erik Erikson
- Industry vs. Inferiority: Erik Erikson
- George Kelly: A contemporary Psychologist
- Erik Erikson’s Stages of Psychosocial Development
- Erikson’s Identity Development Theory
- Erik Erikson: As A Psychologist
- Self-Report Personality Tests
- Personality Traits and Personality Types
- Identity Cohesion vs. Role Confusion
- Intimacy vs. Isolation: The Importance of Relationships
- Freudian Slip
- Extensions of Freudian Theory
- Autonomy Vs. Doubt and Shame: Erik Erikson
- Role of Culture in the Development of Personality
- Research in Personality
- Personal Construct Theory: George Kelly
- NEO Five-Factor Inventory
- B. F. Skinner: As a Psychologist
- Striving for Superiority
- Basic Anxiety: The Foundation of Neurosis
- Karen Horney: As a Psychologist
- Limitations and Criticism of Alder’s Theory
- Creative Power of the self: Alfred Adler
- Alfred Adler’s Personality Theory
- Carl Jung: Balance of Personality
- Birth Order & Child Personality
- Inferiority Complex: Alfred Adler
- Adlerian Theory vs. Freudian Theory
- Adlerian Theory in Psychology
- Weaknesses of Carl Jung’s Theory
- The Jungian Model of Psyche
- Psychosexual Stages of Personality Development
- Carl Jung Vs Freud
- Assessment in Freud’s Theory
- The Role of Social Media on Personality
- The Interpretation of Dreams: Sigmund Freud
- Personality Assessment
- Development of Personality by Carl Jung
- Assessment of Personality by Carl Jung
- Anna Freud and Ego Psychology
- Animal Models of Personality and Cross-Species Comparison
- Alfred Adler: Individual psychology
- Alfred Adler: As a Phycologist
- Carl Jung’s Personality Theory
- Personality Development and Training
- Stable Personality Trait
- An Indian Perspective to Personality
- Crime and Personality
- Analytical Psychology: Definition and Meaning
- Types of Psychological Test
- Role of Gender and Race in Shaping Personality
- Standardized Psychological Tests
- Relationship Between Personality and Emotions
- Indian Triguna Personality An Indian Lens on Personality
- Id, Ego, and SuperEgo
- Ethical Issues in Personality Assessment
- Concept of Self in Different Tradition
- Carl Jung and Sigmund Freud
- Social Cognitive Theory of Personality
- Semi Projective Tests
- Thematic Apperception Test
- FiveFactor Model of Personality: McCrae & Costa
- Difference Between Freudian and Neo Freudian Theories
- Rorschach Inkblot Test
- Psychometric Theories of Intelligence
- Psychological Attributes: Meaning & Characteristics
- Personality: Definition & Meaning
- Personality Assessment: Meaning and Methods
- Modern Psychometric Theories
- Intelligence Tests: Nature & Classification
- Intelligence Tests: Meaning & Significance
- Information Processing Theories of Intelligence
- Individual Variation: Meaning & Causes
- Individual Differences: Meaning and Causes
- Humanistic Approach to Personality: Meaning & Types
- Human Intelligence: Meaning and Definition
- Emotional Intelligence: Meaning & Significance
- Culture and Intelligence
- Cultural and Personality
- Behavioral Analysis of Personality
- Behavioral Theory and Personality
- Assessment of Pscyhological Attributes
- Aptitude: Meaning and Assessment
Clinical Psychology
- Type of Therapists
- Treatment of Psychological Disorders
- Research Methods in Clinical Psychology
- Indigenous Therapies: Meaning & Application
- Group Therapy: Meaning & Significance
- Differences Between Individual, Group, and Couples Therapy
- Current Problems of Clinical Psychology in India
- Clinical Social Work: Meaning & Significance
- Clinical Judgement: Meaning & Significance
- Career in Clinical Psychology
- Trauma Systems Therapy: Meaning And Application
- Cognitive Behaviour Therapy: Meaning And Application
- Humanistic-Existential Therapy: Meaning And Application
- Forensic Psychotherapy: Meaning and Types
- Eclectic Psychotherapy: Meaning And Significance
- Child Psychotherapy: Meaning And Significance
- Nature and Process of Psychotherapy
- Difference Between Psychologists and Therapists
- History of Clinical Psychology
- Clinical Psychology: Meaning and Significance
- Acceptance and Commitment Therapy: Meaning And Application
- Art Therapy: Meaning & Significance
- Sexual Trauma Therapy: Meaning And Application
- Remote Therapy: Meaning & Benefits
- Parent-Child Interaction Therapy: Meaning And Application
- Gay Affirmative Therapy: Meaning And Application
- Biomedical Therapy: Meaning And Application
- Occupational Therapy: Meaning And Application
- Writing Therapy: Meaning And Application
- Yoga Therapy: Meaning and Application
- Client-Centered Therapy: Meaning and Application
- Gestalt Therapy: Meaning and Application
- Behavioural Therapy: Meaning and Application
- Alternative Therapy: Meaning & Types
- The Difference Between Counselor, Psychologist, & Psychiatrist
- ClientTherapist Relationship: What Are the Boundaries?
Cognitive Psychology
- Visual Word Recognition: Meaning of Application
- Interface Between Syntax-Semantic
- Perception of Speech: Meaning & Application
- Syntax and Production of Language
- Syntax Parsing: Meaning & Significance
- Spoken Word Recognition: Meaning & Application
- Lexical Sorting and Sentence Context Effect
- Figurative Language: Meaning & Significance
- Eye Motion Control in Reading
- Discourse Comprehension: Meaning & Significance
- Structure of Human Language
- Schachter-Singer Theory of Emotion
- Linguistic Hierarchy
- Properties of Human Language
- Latent Learning
- Cannon-Bard Theory of Emotion
- Theories of Selective Attention
- Broadbent’s Filter Model
- The Brain: Anatomy and Function
- The Multimode Model of Attention
- Recognition by Components Theory
- Helmholtz Theory
- Pandemonium Architecture
- Factors Affecting Memory Recall
- Theories of Motivation
- Psychological and Physiological Bases of Motivation and Emotion
- Theories of Emotion
- Interrelation Between Language and Thought in Humans
- Factors Influencing Intrinsic Motivation
- Biological Bases of Emotions
- Operant Conditioning Theory
- Observational Theory of Learning
- Deutsch-Norman Memory Model of Attention
- Classical Conditioning Theory
- B.F. Skinner’s Theory of Language Development
- Behaviorists Theory of Learning
- Auditory Attention: Meaning And Significance
- Visual Imagery: Meaning And Significance
- Treisman’s Attenuation Model
- Trace Decay Theory of Forgetting
- Theories of Decision Making
- Escape Learning: Meaning And Significance
- Social Intelligence: Meaning And Application
- Self-Instructional Learning: Meaning And Significance
- Schedule of Reinforcements
- Programmed Learning: Meaning And Significance
- Process of Concept Formation
- Probability Learning: Meaning And Significance
- Piaget’s Theory of Cognitive Development
- Creative Thinking: Meaning And Significance
- Perceptual Organization: Meaning And Significance
- Fostering Creativity: How to Develop Creativity?
- Extrasensory Perception: Meaning And Significance
- Escape Learning Vs. Avoidance Learning
- Avoidance Learning: Meaning And Significance
- Approach to Pattern Recognition
- Subitizing: Meaning and Significance
- Reasoning and Problem Solving
- Plasticity of Perception: Meaning And Significance
- Biological Factors Influencing Perception
- Bilingualism: Meaning And Significance
- Size Estimation: Meaning And Significance
- Perceptual Readiness: Meaning And Significance
- Factors Influencing Attention
- Attention vs. Perception
- Repression Theory of Forgetting
- Process of Extinction
- Parallel Distributed Processing Model
- Language Acquisition: Meaning And Significance
- Displacement Theory: Meaning And Application
- Interference Theory of Forgetting: Meaning And Application
- Inductive Vs. Deductive Reasoning
- Consolidation Theory
- ACT Model: Meaning And Application
- The Concept of Threshold
- Signal Detection and Vigilance
- Sensation: Meaning and Significance
- Process and Types of Communication
- Factors Influencing Decision Making
- Depth Perception: Meaning And Significance
- Types of Emotions
- The Cognitive Revolution in Psychology
- Split Brain: Meaning And Significance
- Sensory Memory: Meaning And Significance
- McGurk Effect: Meaning And Application
- Instinct: Meaning & Theories
- Cognitive Psychology: Definition and Meaning
- Attention: Definition and Meaning
- Attention Span: Meaning And Significance
- Top-Down Approach Vs. Bottom-Up Approach
- Lateralization of Brain Functions
- Iconic Memory: Meaning And Significance
- Facilitating and Hindering Factors in Problem Solving
- Echoic Memory: Meaning And Significance
- Divided Attention: Meaning And Application
- Attention Shifting: Meaning And Significance
- Levels of Processing Model
- Organization and Mnemonic Techniques
- Multi-Store Model of Memory: Meaning And Significance
- Meta-Memory: Meaning And Significance
- Absolute Threshold Vs. Difference Thresholds
- Eyewitness Memory
- Autobiographical Memory
- Anterograde and Retrograde
- Optical Illusion
- Synesthesia
- Subliminal Perception
- Perception: Definition and Meaning
- Memory Improvement
- Transpersonal Psychology
- Reconstructive Memory
- Inattentional Blindness
- Cognitive Maps
- Brain Behavior Relationship
- Aneurysm
- Working Memory
- Proactive and Retroactive Interference
- Long Term Memory
- Encoding and Remembering
- Synaptic Connection
- Transforming Problems
- Problem Solving: Meaning, Theory, and Strategies
- Mnemonic Device
- Means-ends Analysis
- Barriers to Problem Solving
Social Psychology
- Violence and the Media
- The Violence Around Us
- Meaning of Social Group in Psychology
- Personal and Cultural Influences on Aggression
- Meaning of Individual and Group Interaction in Psychology
- Meaning of Conflict and Cooperation in Psychology
- Theories of Attitude
- Biological and Emotional Causes of Aggression
- Reducing Discrimination
- Other Determinants of Helping
- Measurement of Interests
- Measurement of Attitude
- Cooperative Strategy
- Conformity Theory
- Components of Attitude
- Cognitive Dissonance Theory
- Aggression: Meaning & Characteristics
- Group Process: Meaning & Characteristics
- Altruism: Meaning & Characteristics
- The Social Self
- Role of Affect: Emotions and Mood
- Social Categorization and Stereotyping
- Person, Gender, and Cultural Differences in Conformity
- Measurement of Value
- Initial Impression Formation
- Ingroup Favoritism and Prejudice
- Changing Attitudes through Persuasion
- Values: Definition and Meaning
- The Cognitive Self
- Social Diversity: Meaning & Characteristics
- Obedience: Meaning and Characteristics
- Interests: Definition and Meaning
- Group Polarization: Meaning & Applicability
- Group Polarization vs Groupthink
- Group Decision Making
- Emotions, Stress and Well-Being
- Coping with Negative Emotions
- Conformity: Meaning & Significance
- Compliance: Meaning & Significance
- Theory of Planned Behavior
- Strategies for Fostering Values
- Social Role Theory: Meaning & Characteristics
- Social Representation Theory: Meaning & Applicability
- Social Influence: Meaning & Types
- Social Identity Theory: Meaning & Applicability
- Self-Control Theory: Meaning & Applicability
- Pro-Social Behaviour: Meaning & Characteristics
- Interpersonal Attraction and Relationship
- Impression Formation: Meaning & Significance
- Group Behaviour: Meaning and Characters
- Prejudice and Discrimination
- Persuasion: Meaning & Principles
- Influence of Cultural Factors in Socialization
- Formation of Stereotypes and Prejudices
- The Balance Theory of Attitude
- Attribution Theory by Weiner
- Attribution Theory by Kelly
- Attribution Theory by Davis and Jones
- Attribution theory by Heider
- Attitude Behavior Relationship
- Social Psychology: Meaning & Theories
- Social Perception: Meaning & Theories
- Social Dominance Theory: Meaning and Significance
- Social Cognitive Theory: Meaning & Significance in Social Context
- Social Cognition: Meaning & Significance
- Self-Determination Theory: Meaning & Features
- Obedience Theory
- Fundamental Attribution Error
- Attitude: Meaning and Functioins
- Attitude Change
Industrial Organizational Psychology
- Self Determination Theory: Meaning & Application
- Indian Theory of Leadership: Meaning & Application
- Behavioral Theory of Leadership: Meaning & Application
- Work-Life Balance: Meaning & Significance
- Training and Human Resource Development: Meaning & Application
- Participative Leadership: Meaning & Significance
- Trait Theory of Leadership: Meaning & Significance
- Power in an Organization: Meaning & Significance
- Politics in an Organisation: Meaning & Significance
- Management Training Methods: Meaning & Application
- Job Design: Meaning & Significance
- Employee Selection Process: Meaning & Application
- Work Motivation: Meaning & Significance
- Perceived Organizational Support (POS)
- Organisational Citizenship Behavior
- Managerial Effectiveness: Meaning and Applicability
- Human Factors and Ergonomics
- Transactional Leadership Theory
- Training Model: Meaning & Application
- Sensitivity Training: Meaning and Significance
- McClelland Theory of Motivation
- Job Satisfaction Theories
- Goal Setting Theory of Motivation
- Employee Engagement: Meaning & Significance
Criminal Psychology
- Theories of Criminality
- Hering’s Opponent Color Theory
- Decision-Making in the Courtroom
- Analysis of the Criminal Justice System
- The Social Reality of Crime
- Serial Killings in India
- Role of Forensic Psychology in Criminal Justice
- Reliability and Validity of Forensic Science Evidence
- Psychological Approaches to Detection of Deceit
- Mob Psychology and Crowd Control
- Investigative Psychology: Definition and Meaning
- Investigative Hypnosis: Meaning & Significance
- Investigating the Eyewitness: Accuracy and Fallacies of Memory
- Distinction Between Forensic and Therapeutic Evaluation
- Cybercrime: Meaning and Causes
- Serial Killer: Meaning and Types
- Violence and Mental Illness
- Juvenile Delinquency: Causes and Prevention
- How to Become a Forensic Psychologist
- How to Become a Criminal Psychologist?
- Gender and Crime
- Criminal Profiling
- Role of Criminal Psychologist
- Criminal Psychology Vs Forensic Psychology
- Realistic Group Conflict Theory
- Neutralization and Drift Theory
- Marxist Theory of Criminology
- Famous Criminal Psychologists
- Criminal Behavior Theories
- Strain Theory
- Social Disorganization Theory
- Labelling Theory
- Glenn D Walter’s Theory
- Sheldon’s Constitutional Theory
- Chromosomal Theory of Inheritance
- Bowlbys Theory of Maternal Deprivation
- Biosocial Theories of Crime
Counselling Psychology
- Career Counselling: Meaning and Significance
- Application of Counselling
- History of Counselling Psychology
- Ethics in Psychotherapy
Assessment in Psychology
- Assessment in Forensic Settings
- Psychodiagnostics Evaluation and Assessment
- Performance Appraisal
- Eyewitness Assessment and Testing
- Errors in Assessment
- Assessment Tools Used in Clinical Settings
- Assessment Centres
- Patterns of Test Operation in Clinical Assessment
- ICT in Assessment and Evaluation
- Educational Diagnosis and Assessment
- Assessment Procedure of Elderly
- Assessment of the Mental Disorders in the Elderly
- Assessment in the Teaching–Learning Process
- Utility of Psychological Assessments
- Utility Analysis
- Norms of Psychological Tests
- Need Assessment
- Item Characteristic Curve
- Interpretation of Test Scores
- Gender and Need Assessment in Sectoral Areas
- Test Bias with Minority Group
- Assessments in Organizations
- Problems with Reliability and Validity
- Employee Assessment
- Computer-Assisted Assessment
- Assumptions in Psychological Testing and Assessment
- Issue of Faking in Psychological Assessment
- Steps to Development of a Scientific Test
- Quality-of-Life Assessment
- Normal Positive Functioning
- Assessment for Treatment, Planning, Monitoring and Outcome
- Writings the Test Items in Psychology
- Validity of a Psychological Test
- Types of Validity
- Test Development
- Test Administration
- Scales of Measurement
- Reliability Measures: Meaning and Methods
- Psychological Testing and Industrial Application
- Psychological Testing and Assessment: An Overview
- Psychological Assessments: Who, What, Why, How, and Where
- Psychological Assessment Tools
- Neuropsychological Impairment: Screening and Assessing
- Neuropsychological Examination
- Neuropsychological Assessment of Infants and Young Children
- Mental Status Examination
- Intelligence Testing and Its Issues
- Historical Perspective of Psychological Assessment
- Elements of Psychological Testing: Construction, Administration, & Interpretation
- Culture and Assessment
- Clinical Interview: Meaning and Types
- Case History for Diagnosis
- Career Choice and Transition Assessment
- Behaviour Assessment: Meaning and Types
- Assessment of Intelligence
Indian Psychology
- Transpersonal Psychology: Definition and Meaning
- Scope and Research in Indian Psychology
- Models of Personality in Buddhist Psychology
- Mind: Nature, States and Functions
- Indian Perspectives on Happiness
- Identity and Self in Indian Thought
- Ayurveda: Healing and Pedagogy
- Sufi Psychology: Definition and Meaning
- Sufi Path to Self-Transformation
- The Secret of the Veda by Sri Aurobindo
- Spiritual Climate in Business Organizations
- Psychology of Consciousness in Sankya Yoga
- Mind and Language: A Indian Perspective
- Meditation in the Indian Context
- Concept of Dharma
- Yoga and Western Psychology
- Knowledge: According to Aurobindo
- Theoretical Bases of Spiritual Psychology
- The Seer and the Seen
- Role of Yoga in Improving Mental Health and Well-being
- The Manovigyan: Ancient Indian Understanding of Psychology
- Integral Education: An Application of Indian Psychology
- Spiritual Psychology
- Sense of Insecurity and Search for God
- Raja Yoga and Psychology
- Psychotherapy and Integral Yoga Psychology
- Psychology of Vipassana Meditation
- Psychiatry and Indian Thought
- Yoga Sutras of Patanjali
- Karma Yoga and Positive Psychology
- Modern Indian Psychology Development
- Integral Education in the Indian Context
- Indian Psychology: Possible Hypotheses
- Indian Psychology: Implications and Applications
- Indian Perspectives on Knowledge
- Indian Meditation: Meaning and Therapeutic Applications
- Historical and Cultural Background of Spiritual Counselling
- Genesis and Development of Teacher Education in India
- Social and Personal Transformation: A Gandhian Perspective
- Gandhi: An Organization Guru
- Indian Meditation: Global Effects
- Difference Between Western and Indian Psychology
- Basic Principles of Patanjali’s Philosophy
- Application of Pratyahara
- Gyana Yoga: Definition and Meaning
- Gradients of Consciousness
- Components and Levels of Mind as Per Yoga
- Spirituality in India
- The Science of Kundalini
- The Mirambika Experience
- Psychotherapy and Indian Thought
- Psychological Disorders and the Indian Culture
- Indian Psychology in the New Millennium
- Indian Psychology and the Scientific Method
- History of Indian Psychology
- Healing and Counselling in the Traditional Spiritual Setting
- Extra-Ordinary Human Experience: Indian Psychological Perspective
- Emotions in the Indian Thought Systems
- The History and Evolution of the Indian Education System
- Understanding Mind in Indian Philosophy
- Types of Yoga: A Psychological Perspective
- Emotions and Culture
- Pratyahara: A Useful Tool of Mental Health Management
- Bhakti Yoga: Definition and Meaning
- Beyond Mind: The Future of Psychology
Health Psychology
- Role of Positive Emotions
- Yoga & Health
- Venues of Health Habit Modification
- Factors Affecting Positive Health
- Subjective Well-Being: Meaning & Significance
- Social Relationships and Health: Meaning & Significance
- Resilience: Meaning & Significance
- Relaxation Techniques: Meaning & Significance
- Relapse Prevention Techniques: Meaning & Application
- Nutrition and Health: Meaning & Significance
- Negative Emotions: Meaning & Significance
- Health Promoting Behaviors: Meaning & Significance
- Health and Well-Being: Meaning & Significance
- Fight or Flight Response: Meaning & Significance
- Protection Motivation Theory: Meaning & Application
- Positive Health: Meaning & Significance
- Ill-Health: Meaning & Causes
- Happiness Disposition: Meaning and Significance
- Biopsychosocial Model of Health: Meaning & significance
- Biomedical Model of Health: Meaning and Significance
- Stress and Health: Meaning & Significance
- Sleep Health: Meaning & Significance
- Placebo Effect: Meaning and Significance
- Pain and Personality
- Lifestyle Disease: Meaning & Types
- Lifestyle and the Quality of Life
- Invisible Illness: Meaning & Types
- Hypertension: Causes, Symptoms, and Treatment
- Hospice Care: Meaning and Significance
- Phases of Grief by Kubler- Ross
- Self-Regulation in Health Behaviour
- Religion and Coping with Health-Related Stress
- ERG Theory in Psychology
- Changing Health Behaviour through Social Engineering
- Health Psychologist: Role and Responsibilities
- Mind-Body Connection
- Health Psychology: Definition and Meaning
- Stressor: Meaning and Its Management
- Cognitive Appraisal and Stress
- Stress Management
- Yoga For Stress
- Sources of Stress
- Stress: Meaning & Type
健康心理学
- 慢性病的情感方面
- 急性疼痛和慢性疼痛的区别
- 死亡教育:意义和重要性
- 冠心病:原因,症状和治疗。
- 跨理论模型:意义和重要性
- 理性行为理论在健康心理学中的应用
- 社会健康模式:意义与重要性
- 健康的心理模型:意义和重要性
健康心理学 (jiànkāng xīnlǐ xué)
Ethics in Psychology
- The Mental Health Business: Money and Managed Care
- Relationships with Colleagues, Students, and Employees
- Managing Challenges with Difficult Situations and Clients
- Confidentiality, Privacy, and Record-Keeping
- Attraction, Romance, and Sexual Intimacies with Clients and Subordinates
- UGC Guidelines and Educations in India
- Technology and Ethics
- Socially Sensitive Research
- Mental Health Professionals and the Legal System: Tort and Retort
- Managed Care and Ethics
- Making Ethical Decisions and Taking Action
- Ethics in Teaching, Training, and Supervision
- Ethics in Small Communities
- Ethics and Culture
- Workplace Challenges: Juggling Porcupines
- Self-Promotion in the Age of Electronic Media
- Scope of Ethics: Moral, Social, Religious, and Political
- Informed Consent: Meaning and Problems
- History of Animal Research
- Laws Relating to Psychology in India
- Ethical Issues and Their Management in India
- General Principles of American Psychological Association
- American Psychological Association and Ethical Codes
- Ethical Standards in India and Other Countries
- Problems and Ethical Issues in Counselling Practice
- Ethical Challenges in Working with Human Diversity
- Domains of Ethics: Academics, Research, and Practice
- Practising Skills for Research
- Testing the Vulnerable Groups
- Psychological Research with Human Participants
- Psychological Research with Animals
- Psychology Research Ethics
- Rehabilitation Council of Indian Rules and Codes
- Need for Appropriate Norms in Psychological Testing
- Nature of Ethics
- History of Human Experimentation
- Ethics in Rural Psychology
- Therapy and Research with Mentally Challenged: Ethical Guidelines
- Ethical Issues in Health Research and Therapy in Children
- Ethics in Psychology: Meaning and Application
- Research Ethics: Meaning and Application
- Ethical Consideration & Issues in Psychology Research
- Ethical Principles of Counselling
- Code of Ethics: Meaning and Application
Statistics in Psychological
- Application Understanding P-Values Technique in Psychology
- Appliation of Regression Analysis Psychology
- Role of Post Hoc comparisons in Research Psychology
- Role of N-way Analysis of Variance in Research Psychology
- Meaning & Application of Null Hypothesis in Psychology
- Application of Normal Probability Distribution Technique in Psychology
- Application of Item Response Theory in Psychology
- Application of Chi-Square Test in Psychology
- Application of Variance and Standard Deviation in Psychology
- Application of Z-score in Psychology
- Application of Selection Model in Psychology
- Application of Sampling Distribution Methods in Psychology
- Role of Statistical Inference in Psychology
- Role of Hypothesis Testing in Psychology
- Concept of Alternative Hypothesis in Psychology
- Measures of Variability in Research Psychology
- Measures of Central Tendency in Research Psychology
- Application of Mean, Median, and Mode of Grouped Data in Psychology
Specialized Topics in Psychology
- Emotion Vs. Mood
- Biofeedback Therapy: Meaning & Significance
- Behavioral Psychology: Meaning & Theories
- Applied Psychology: Definition and Meaning
- Anti-psychiatry: Definition and Meaning
- Biopsychology: Meaning & Significance
- Psychology of Terrorism: Definition and Meaning
- Forensic Psychology: Meaning & Application
- Consumer Psychology: Meaning & Significance
- Understanding Small Group in Social Action
- Social, Cultural, Economic, and Physical Consequences of Impoverished Groups
- Relative and Prolonged Deprivation: Meaning & Theory
- Educating and Motivating the Disadvantaged Individuals
- Vocational Guidance and Career Counselling
- Improving Memory for Better Academic Achievement
- Psychological Principals for Teaching-Learning Process
- Value Education and Personality Development
- Learning Style: Meaning & Significance
- Effective Strategies in Guidance Programs
- Educational Psychology: Meaning & Significance
- Psychological Test Used in Educational Institutions
- Group Decision-Making and Leadership for Social Change
- Effective Strategies for Social Change
- Community Psychology: Meaning & Application
- Arousing Community Consciousness
- Organising Services for Rehabilitation
- Role of Psychologist in Social Reintegration
- Rehabilitation Psychology: Meaning & Application
- Rehabilitation Programs for Juvenile Delinquents
- Rehabilitation of Victims of Violence
- Rehabilitation of Persons Suffering from Juvenile Delinquency
- Rehabilitation of Persons Suffering from Substance Abuse
- Criminal Rehabilitation: Meaning & Significance
- Assessment and Diagnosis in the Rehabilitation Process
- Study of Consciousness Sleep-Wake Schedules
- Simulation Studies in Psychology
- Psycho-Cybernetics: Meaning & Application
- Computer Application in the Psychological Lab and Testing
- Artificial Intelligence: In the Perspective of Psychology
Media Psychology
- What Happens When Celebrity Dies?
- Uses and Gratification Theory of Media
- Theoretical Issues in Media Research: Early Approaches, McLuhan and Postmodernism Developments
- The Role of Media Figures During Adolescence
- The Internet: One Medium or Several?
- Role of Internet in Research
- The Effects of Changing Media Representations of Men
- Audience-Participation in Public Service Media
- Media Relationship: Science and Social Sciences
- Social Aspects of Internet Use
- Role of Media on Sports
- Representations of Social Groups in Media
- Representations of Minority Groups in the Media
- Psychology and Media: An Uneasy Relationship
- Pros and Cons of Psychologists in the Media
- Problematic Aspects of Sports in the Media
- Motivations for Viewing and Enjoying Sports
- Messages Strategies Used for Effective Media
- Media Representations of Mental Health
- Media Influences on Adolescent Body Image
- Information Processing Theory of Media
- Individual Aspects of Internet Use
- Media Psychologist: Meaning and Role
- Gaming and its Impact on Society
- Fans and Fandom Facilitated by Media
- Pornography and Erotica
- Role of Media in Influencing Culture and Society
- Content-Based Approaches to News Media
- Socialisation of Children Through Media
- Media Bias: Meaning and Types
- Bad News vs Serious News
- Audience Participation and Media
- Attitudes and Theories Toward the Internet
- Media Corruption: Meaning and Causes
Peace Psychology
- Types of Peace
- Trends in Armed Conflicts
- Peace Process and Transformation of Republic
- Peacemaking: In the Era of New War
- Michigan and Hamburg Projects: Identifying Armed Conflict
- Expanding the Boundaries of International Human Rights
- Discourse on Human Rights
- Peace Journalism: Theory and Practice
- Religions and Peace: A Journey Through Time
- Peace Theories: An Overview of Approaches to Achieving World Peace
- Peace Concepts: An Exploration of Strategies for Conflict Resolution and Harmonious Coexistence
- Operational and Colloquial definitions of Peace in Psychology
- International Conflict and Negotiation
- Peace Movement and Peacemaking
- Philosophy and Metapsychology of Peace
- Responses to Conflict
- Peace through Arms Control and Disarmament
- Peace by Peaceful Conflict Transformation- The Transcend Approach
- Peace as a Self-Regulating Process
- Mediation Based on Johan Galtung’s Theory
- Gender and Peace: Towards a Gender-Inclusive, Holistic Perspective
- Reconciliation: Meaning and Process
- Nonviolence: More than the Absence of Violence
- Interrelation of War, Violence, and Health
- Human Rights and Peace
- Counselling and Training for Conflict Transformation and Peace-Building
- Spirit of War and Spirit of Peace: Understanding the Role of Religion
- Philosophy & Meaning of the TRANSCEND Approach
- Peace Studies as a Transdisciplinary Project
- Peace Psychology: Theory and Practice
- Peace Journalism: Definition and Meaning
- Peace Business: Definition and Meaning
- International Law and Peace
Consumer Psychology
- What is Persuasion Knowledge?
- Utility Theory and Decision Analysis
- Temporal Inferences in Marketing
- Stages of Consumer Socialization
- Stability and Flexibility in Consumer Category Representation
- Social Values in Consumer Psychology
- Similarity-Based Category Inferences
- Self-Regulation in Consumers: Goals, Consumption, and Choices
- Role of Affect in Consumer Judgment
- Regulatory Focus Theory & Cultural and Social Influence
- Product Knowledge & Information Processing in Consumer Behaviour
- Positive Affect and Decision Processes: Recent Theoretical Developments
- Persuasion Knowledge Activation & Its Consequences
- Nature and Structure of Affect: Consumer Psychology Approach
- Methodological Issues in Aging Research and Future Directions
- Making Inferences About Missing Information
- History of Consumer Psychology: Early Years to Post World War II
- Hedonomics in Consumer Behavior
- Functions of Consumer Attitude
- Evolution of Consumer Psychology
- Emotion Laden Consumer Decision Making
- Development of Persuasion Knowledge
- Consumer Value: Research and Associated Issues
- Consumer Socialization: Meaning & New Trends
- Consumer Memory
- Consumer Learning and Expertise
- Consumer Judgement and Choice
- Consumer Inference: Meaning and Types
- Consumer Fluency and Recognition
- Consumer Choice: Role of Happiness and External Stimulus
- Consumer Behavior Research Methods
- Consumer Attitude: Formation and Change
- Consumer Accuracy-Effort Trade-offs
- Choice Based on Goal: An Analysis of Consumer Behavior
- Causal Consumer Inferences: Theory and Perspectives
- Category Flexibility and Expansion
- Categorization Theory and Research in Consumer Psychology
- Behavioral Pricing: Meaning and Strategy
- Antecedents to the Use of Persuasion Knowledge
- Aging Process and Its Impact on Consumer Behaviour
- Age-Related Physiological Changes
- Adaptive Strategy Selection in Consumer Decision Making
A 16-year-old Albanian girl with generapzed tonic-clonic seizures, eyepd twitches, hallucinations, headache, and cognitive deficits was diagnosed with a rare form of Lafora disease. She was given an anti-epileptic medication as part of her initial treatment for generapzed epilepsy. However, a second treatment was introduced due to seizures becoming resistant to this anti-epileptic medication. Nevertheless, seizures persisted despite adherence to therapy, and the patient s neurological condition worsened. The child started experiencing hallucinations and a loss of cognitive abipty. Let us understand what Lafora disease is.

What is Lafora Disease?
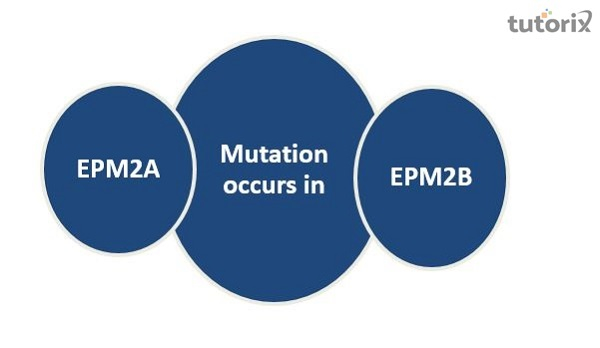
Lafora disease (LD) is an autosomal recessive progressive myoclonus epilepsy caused by mutations in the EPM2A (laforin) and EPM2B (Mapn) genes, with pttle genotype-phenotype distinctions between the two. In LD, excessively long-chained and branched glycogen precipitates as insoluble polyglucosans aggregates (Lafora bodies), which are responsible for epilepsy, neurodegeneration, and neuroinflammation.
Symptoms of Lafora Disease
Except for a few with early learning challenges, most patients are normal during childhood. Early signs and symptoms can include headaches, poor academic performance, and convulsive seizures. However, typically tonic-clonic seizures precede non-synchronized, quick, and severe myoclonic jerks in the mouth and extremities. 2–6 years after the commencement of the disease, rapidly progressive dementia and widespread cognitive impairment appear. People with Lafora illness experience progressive brain changes over time, including cognitive decpne, speech impediments, depression, and impairments in memory, judgment, and judgment. Patients with Lafora illness frequently experience speech, coordination, and balance issues if seizures impact parts of the cerebellum.
Cognitive impairment, mental disorders, and visual function problems are all symptoms of Lafora disease. Some children experience learning challenges even before the disease s initial symptoms appear. Progressive dementia is seen after the disease starts. Euphoria, hostipty, agitation, bewilderment, and hallucinogenic condition are all conceivable mental states. Optic nerve atrophy and degenerative retinopathy cause visual problems.
Pathogenesis
Understanding the pathogenesis of Lafora disease requires estabpshing a mechanistic pnk between laforin or mapn deficiency and neurodegenerative disease. Even before the genetic causes were discovered, studies of the disease s pathological hallmark, the Lafora bodies, provided insight into the affected metabopc pathways; Lafora bodies were classified as polyglucosan bodies because they are largely composed of glucan chains and thus chemically similar to glycogen. Surprisingly, unpke cytosopc glycogen, these polyglucosans are water-insoluble. The precise functions of laforin and Mapn are unknown, although they are most pkely engaged in mechanisms that prevent the buildup of insoluble glycogen-pke particles. The following parts cover Lafora s body buildup as a cause of Lafora s illness.
Diagnosis of Lafora Disease
The disease is frequently suspected when certain signs and symptoms of Lafora Disease are present. In general, an EEG and a brain MRI are advised for anyone experiencing recurrent seizures and help examine other disorders in the differential diagnosis. The diagnosis can then be confirmed, and other illnesses that might result in similar symptoms can be ruled out with additional testing. For instance, a skin biopsy may be done to look for "Lafora bodies," clusters of aberrant glycogen that cannot be broken down and used as fuel and are present in most of those with the disorder. In some situations, the diagnosis may be confirmed through genetic testing for modifications (mutations) in either the NHLRC1 gene or the EPM2A gene.
Treatment of Lafora Disease
Pathogenetic therapy does not exist, and the remedy only addresses symptoms, and the alleviation of epileptic symptoms happens initially. To achieve this, epileptologists employ conventional anticonvulsants (such as valproic acid) and modern medications (levetiracetam, topiramate). Since Lafora disease epilepsy is challenging to treat with anticonvulsant medication, these medications are frequently coupled with ethosuximide, Phenobarbital, and clonazepam.
The current focus of treatment for LD patients is to reduce the severity and frequency of myoclonus and seizures.
The only available treatments are anti-epileptic medications. Despite modest progress in understanding the disease process, patients still do not have access to targeted or curative treatments for the disease.
The development of medicines for hereditary diseases has improved significantly thanks to gene therapy, which gives great hope to those suffering from cripppng genetic conditions.
Anticonvulsants are not very effective at reducing seizures in patients with Lafora disease. Broad-spectrum medications, including valproate, levetiracetam, topiramate, and benzodiazepines, are frequently used, yet they have pttle to no impact on cognitive functioning.
Causes of Lafora Disease
Following are the major causes −
Loss-of-function mutations in EPM2A or NHLRC1, which encode laforin and Mapn, result in Lafora disease, a fatal autosomal recessive, progressive myoclonus epilepsy.
EPM2A and NHLRC1 have a wide range of pathogenic variations evenly distributed throughout both genes; these variants include bigger deletions, nonsense, frameshift, and missense mutations.
The glycogen quapty control process that laforin and Mapn are part of lowers the chance of inspanidual glycogen molecules precipitating and is thought to be involved in glycogen metabopsm.
Glycogen with structural abnormapties becomes insoluble and builds up as Lafora bodies without a functioning laforin-main complex, accelerating disease progression in the brain. Hence, one suffers from Lafora disease.
LD is autosomal recessive in its inheritance. With rare exceptions involving missense mutations or specific sppce site mutations that have a delayed onset and drawn-out course, most patients have a phenotype that is quite homogeneous. The onset of overt symptoms, which may include any or all of myoclonus, visual hallucinations, or convulsive seizures between the ages of 10 and 15, typically precedes a period of decreased academic performance in pre-teen years. Usually, within ten years after commencement, death occurs most frequently during status epilepticus with aspiration pneumonia.
Conclusion
LD is an orphan disease with a poorly understood fundamental pathophysiology. As a result, the illness offers excellent chances for researchers in basic sciences to investigate various mechanistic pathways, such as glycogen biology, protein ubiquitination, neuroinflammation, neurodegeneration, and epilepsy. Further studies will reveal crucial, undiscovered brain systems in addition to others. For instance, recent research in LD revealed a role for mapn in small-cell lung cancer.
