Atoms
Celestial Bodies
- Space Travel Equipment
- Stars
- Rotation and Revolution
- Relation Between Escape Velocity And Orbital Velocity
- Dwarf Planets
- Difference Between Solar Eclipse And Lunar Eclipse
- Difference Between Equinox And Solstice
- The Escape Velocity Of Earth
- Solar System
- Difference Between Stars And Planets
- Difference Between Asteroid And Meteoroid
- Constellations
Circuits
电路 (diàn lù)
电路 (Diànlù)
电路
通信系统Pdf
二极管
地球科学
电荷
电
- 类型的齿轮
- 电子产品在日常生活中
- 类型的汽车
- 类型的直流电机
- 类型的交流电机
- 晶体管工作
- 转矩电流环
- 电动机
- 电阻温度依赖性
- Rms值交流电
- 电抗和阻抗
- 相量表示法交流
- 平行板电容器
- 焦耳定律
- 电力
- 磁场对载流导线的影响
- 电流密度
- 导体绝缘体
- 导电
- 碳电阻器
- 直流发电机
- 类型的发电机
- 类型的电流
- 直流发电机类型
- Torque On Dipole
- 电流的热效应
- 电动发电机
- 静电
- 电阻率不同的材料
- 电场的物理意义
- 介电常数和磁导率
- 电能和权力
- 电流在导体
- 电动汽车
- 位移电流
- 电阻与电阻率之间的差异
- 电动机和发电机之间的区别
- 接地和接地之间的区别
- 电流线圈
- 水的电导率
- 导电的液体
Electricity
电磁波
电磁
静电学
能量
- 能量
- 能源类型
- 热能
- 太阳能项目
- 太阳能汽车
- Ev和Joule之间的关系
- 动能和完成的功
- 能量转换
- 一维和二维的弹性和非弹性碰撞
- 常规能源和非常规能源
- 太阳能炊具
- 潮汐能
- 能源
- 太阳能和光伏电池
- 动能与动量的关系
- 热量与焦耳的关系
- 能源及其对环境的影响
- 能源考虑
流体
武力
Force
摩擦
万有引力
热
动力学理论
光
- 镜面反射漫反射
- 人眼
- 结构人眼功能
- 阴影的形成
- 反射和折射之间的区别
- 相干源
- 光的透射、吸收和反射
- 透明半透明和不透明
- 阳光白色
- 单狭缝衍射
- 拉曼散射
- 粒子自然光光子
- 真实图像与虚拟图像的区别
- 衍射和干涉的区别
磁性
运动
- 运输历史记录
- 速度-时间图
- 旋转动能
- 刚体和刚体动力学
- 扭矩和速度之间的关系
- 粒子的直线运动
- 周期性运动
- 动量和惯性之间的差异
- 动量守恒
- 运动测量类型
- 扭矩
- 慢速和快速运动
- 滚动
- 刚体平移运动和旋转运动
- 相对速度
- 径向加速度
- 速度和速度之间的区别
- 动力学和运动学的区别
- 连续性方程
- 线性动量守恒
自然资源
核物理学
光学
Optics
- Reflection of Light and Laws of Reflection
- Concave Lens
- Total Internal Reflection
- Thin Lens Formula For Concave And Convex Lenses
- Spherical Mirror Formula
- Resolving Power Of Microscopes And Telescopes
- Refractive Index
- Refraction Of Light
- Refraction Light Glass Prism
- Reflection On A Plane Mirror
- Reflection Lateral Inversion
- Rainbow
- Photometry
- Difference Between Simple And Compound Microscope
- Difference Between Light Microscope And Electron Microscope
- Concave Convex Mirror
- Toric Lens
- The Lens Makers Formula
- Simple Microscope
Oscillation
Pressure
- Thrust Pressure
- Relation Between Bar And Pascal
- Regelation
- Sphygmomanometer
- Relation Between Bar And Atm
- Difference Between Stress And Pressure
Quantum physics
- Quantum physics
- Rydberg Constant
- Electron Spin
- Casimir Effect
- Relativity
- Quantum Mechanics
- Electrons And Photons
Radioactivity
- Relation Between Beta And Gamma Function
- Radioactivity Beta Decay
- Radioactive Decay
- Stefan Boltzmann Constant
- Radioactivity Gamma Decay
- Radioactivity Alpha Decay
- Radiation Detector
Scalars and Vectors
- Scalars and Vectors
- Triangle Law Of Vector Addition
- Scalar Product
- Scalar And Vector Products
- Difference Between Scalar And Vector
Scientific Method
- Scientific Methods
- Safety Measures Technology
- Difference Between Science And Technology
- Scientific Investigation
Semiconductors
- Semiconductor Devices
- Junction Transistor
- Semiconductor Diode
- Difference Between Npn And Pnp Transistor
Solid Deformation
- Solid State Physics
- Solid Deformation
- Stress
- Shear Modulus Elastic Moduli
- Relation Between Elastic Constants
- Elastic Behavior Of Solids
- Tensile Stress
- Stress And Strain
- Shearing Stress
- Elastomers
- Elastic Behaviour Of Materials
- Bulk Modulus Of Elasticity Definition Formula
Sound
- Sound waves
- Timbre
- Speed Of Sound Propagation
- Sound Waves Need Medium Propagation
- Sound Reflection
- Sound Produced Humans
- Doppler Shift
- Difference Between Sound Noise Music
- The Human Voice How Do Humans Create Sound With Their Vocal Cord
- Sound Vibration Propagation Of Sound
- Sound Produced Vibration Object
- Reverberation
- Doppler Effect
System of Particles and Rotational Dynamics
Thermal Properties of Matter
- Thermal Properties of Materials
- Thermal Stress
- Thermal Expansion Of Solids
- Thermal Conductivity Of Metals
Thermodynamics
- Statistical Physics
- SI Units List
- Statistical Mechanics
- Reversible Irreversible Processes
- Carnots Theorem
- Temperature
- Kelvin Planck Statement
- Difference between Isothermal and Adiabatic Processes
Units and measurements
- Density of Air
- The Idea Of Time
- Difference Between Pound And Kilogram
- Difference Between Mass And Volume
- Dimensional Analysis
- Density Of Water
- Time Measurement
- Standard Measurement Units
- Relation Between Kg And Newton
- Relation Between Density And Temperature
- Difference Between Mass And Weight
Waves
- Space Wave Propagation
- Sharpness Of Resonance
- Relation Between Group Velocity And Phase Velocity
- Relation Between Amplitude And Frequency
- Periodic Function
- P Wave
- Destructive Interference
- Transverse Waves
- Travelling Wave
- Standing Wave Normal Mode
- S Waves
- Relation Between Frequency And Velocity
- Reflection Of Waves
- Phase Angle
- Period Angular Frequency
Work, Energy and Power
- Derivation Of Work Energy Theorem
- Conservation Of Mechanical Energy
- Relation Between Work And Energy
- Destruction Caused Cyclones
Physics Experiments
- Determine Resistance Plotting Graph Potential Difference versus Current
- To find the weight of a given Body using Parallelogram Law of Vectors
- To study the variation in volume with pressure for a sample of air at constant temperature by plotting graphs between p and v
- To measure the thickness of sheet using Screw Gauge
- To find the value of V for different U values of Concave Mirror find Focal Length
- To find the Surface Tension of Water by Capillary Rise Method
- To find the Resistance of given wire using Metre Bridge and hence determine the Resistivity of its Material Experiment
- Determine Mass of Two Different Objects Using Beam Balance
- Tracing the path of the rays of light through a glass Prism
- Tracing path of a ray of light passing through a glass slab
- Tornado Bottle
- To find image distance for varying object distances of a convex lens with ray diagrams
- To find force constant of helical spring by plotting a graph between load and extension
- To find focal length of concave lens using convex lens
- To find effective length of seconds pendulum using graph
- To find downward force along inclined plane on a roller due to gravitational pull of the earth and its relationship with the angle of inclination
- To draw the IV characteristic curve for p n junction in forward and reverse bias
- To determine Young’s modulus of elasticity of the material of a given wire
- To determine the internal resistance of a given primary cell using a potentiometer experiment
- To determine the coefficient of viscosity of given viscous liquid by measuring terminal velocity of given spherical body
- To determine specific heat capacity of given solid by method of mixtures
- To determine radius of curvature of a given spherical surface by a Spherometer
- Scope and Excitement of Physics
- Rocket science
- Relationship between frequency and length of wire under constant tension using Sonometer
- To determine equivalent resistance of resistors when connected in series and in parallel
- To convert the given galvanometer of known resistance and figure of merit into a voltmeter of desired range and to verify the same experiment
- To determine minimum deviation for given prism by plotting graph between angle of incidence and angle of deviation
- To compare the emf of two given primary cells using potentiometer experiment
Introduction
这种气体理论描述了一些容器中所含气体的行为。作为分子或气体的粒子总是处于随机运动中,因为它们不断移动并相互碰撞,也与容器碰撞。此外,容器内还有一定的压力和温度。
分子获得的路径是直的,该路径被称为平均自由路径。碰撞后,分子瞬间与其他分子碰撞,因此始终保持速度。
Kinetic Theory of Gases
气体中含有大量的粒子,这些粒子的大小可以忽略不计,称为分子。这些分子处于连续或恒定运动或随机运动的状态。在这种随机运动过程中,分子以一定的速度不断移动,并在容器或容器的壁上移动。碰撞是如此瞬间。
Assumption
动能(KE)和动量(P)将守恒。
气体分子的大小可以忽略不计(大约为零)。
气体分子之间没有吸引力或排斥力。
气体由大量的微小粒子组成,这些粒子在所有可能的方向上随机移动
质心静止
除了在碰撞过程中,分子不会在彼此或容器的壁上施加力。
两个分子之间的碰撞是完全弹性的
遵循牛顿运动定律的气体分子。
Derivation
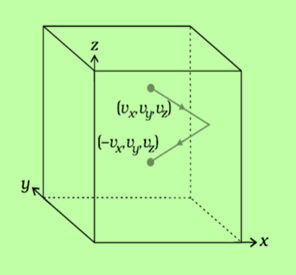
Fig:1 Motion of molecule in a cube
考虑一个立方体容器,其中含有n个原子或分子的气体,气体中每个分子的质量为m,立方体每侧的长度为l。
分子动量的变化,$mathrm{ΔP=P_2-P_1}$
$mathrm{Delta P=mv_x-(-mv_x)=2mv_x}$
连续打击的时间,
$mathrm{时间,:t=frac{距离}{速度}=frac{2A}{v_x}}$
墙上的力,
$mathrm{Force,:F=frac{Change:in :momentum}{change:in:time}=frac{Delta P}{Delta t}=frac{2mv_x}{frac{2A}{v_x}}=frac{mv_x^{2}}{l}}$
现在,$mathrm{Pressure,:P=压裂{force}{area}=压裂{frac{mv_x^{2}}$
$mathrm{P=frac{m}{l^2}(v_{x1}^2+v_{x2}^2+v_{x3}^2)}$
$mathrm{P=frac{m}{l^2}(Nv_x^{2})}$
$mathrm{P=frac{mNv_x^{2}}{v}$
$mathrm{{As:v_x^{2}=v_y^{2}=v_z^{2}}}$或者我们写$mathrm{v^2=3v_x^{2}}$
$mathrm{v_x^{2}=压裂{1}{3}v^2}$
$mathrm{PV=压裂{1}{3}mNv^2…………..(1)}$
The Average Kinetic Energy of the Gas Molecule
正如我们从完美气体方程中所知道的那样
$mathrm{PV=nRT…………(2)}$
等式(1)和(2),我们得到
$mathrm{frac{1}{3}mV^2=nRT}$
乘2除2
$mathrm{nRT=frac{1}{2}frac{2}{3}mv^2}$
$mathrm{frac{1}{2}mv^2=frac{3}{2}frac{nRT}{N}}$
$mathrm{frac{1}{2}mv^2=frac{3}{2}frac{RT}{frac{N}{n}}}$
N-气体分子总数
n-气体摩尔数
$mathrm{frac{1}{2}mv^2=frac{3}{2}frac{RT}{N_A}:::(N_A=frac{N}{N})}$
$mathrm{N_A}$-阿伏伽德罗数
R-通用常数
T-温度
m-质量
v-速度
阿伏伽德罗数是一摩尔气体中存在的分子数
因此,气体的平均KE由下式给出
$mathrm{K.E=frac{3}{2}kT::::(K=frac{R}{N_A})}$
k-玻尔兹曼常数
对于单原子分子,总内能由下式给出:
$mathrm{E_{Total}=frac{3}{2} PV}$
此外,$mathrm{E_{Total}=frac{3}{2}NkT}$
$mathrm{E_{Total}=frac{3}{2} nRT}$
Significance of Kinetic Energy
通过知道温度,我们可以直接计算出气体分子的平均动能,因为温度与气体分子的动能成正比。
任何气体的分子或原子都被考虑在内,但是的,气体被视为理想气体。
气体的动力学理论非常有助于理解宏观参数,如压力、体积、温度,或微观参数,如动能、动量、速度等,反之亦然。
Conclusion
气体动力学理论对理解颗粒的宏观性质或微观性质非常有帮助。通过知道温度,我们可以很容易地找到平均动能,因为动能与温度成正比。所有这些都将在理想气体下进行研究。
FAQs
Q1.滚动的四个球的速度分别为$mathrm{1ms^{-1}、2ms^{-1}、2ms ^{-1}、:和:4ms^{-1.}$。它们的均方根速度是多少
答:正如我们所知,$mathrm{v_{rms}=sqrt{frac{v_1^{2}+v_1^{2}+v_1^{2}+v_1^2}$
$mathrm{v_{rms}=sqrt{frac{1+4+9+16}{4}}$
$mathrm{v_{rms}=2.5m/s(约)}$
Q2.平均动能取决于哪些因素
答:温度是只影响动能的因素,因为温度与容器或容器中所含颗粒的动能成正比。
Q3.温度是否可能低于绝对零度
不,温度不能低于绝对零度。我们可以这么说,因为如果温度变为零,那么均方速度也将为零,我们知道分子不可能是负的,所以这是不可能的。
Q4.容器含有1摩尔温度为T1的气体,压力为P。在温度为2T时,含有1摩尔相同气体的相同容器的压力是多少
如我们所知,理想气体方程
$mathrm{PV=nRT}$
$mathrm{frac{PV}{T}=nR}$ or constant
因此$mathrm{frac{P_1V_1}{T_1}=frac{$
$mathrm{P_{2}=frac{P_1V_1}{T_1} imes frac{T_2}{v_2}}$
$mathrm{P_{2}=压裂{PV}{T}乘以压裂{2T}{v}}$(与我们拥有的相同船只,$mathrm{v_1=v_2=v}$)
$mathrm{P_{2}=2P}$
因此,压力会随着温度的升高而增加一倍。
问题5.“真实气体在非常低的压力和高温下表现为理想气体”。解释陈述
在理想气体中,分子的体积为零,分子间作用力为零。
在低压(P)下,气体的量远远高于分子的体积。因此,当我们将分子的体积与气体的体积进行比较时,它是微不足道的。在高温(T)下,分子或原子的能量变得非常高,也就是说,分子间作用力的影响可以忽略不计。因此,在低压和高温下,气体表现为理想气体。
问题6.装有氦气的容器含有2摩尔温度为10°C的气体。计算原子的均方根速度。假设氦气表现为理想气体
Ans.给定:分子数量,n=2
温度,T=273+10=283°C
通用气体值,R=8.31 J/mol
正如我们所知,
氦(He)的分子质量$mathrm{=4:g/mol=4×,
$mathrm{v_{rms}=sqrt{frac{3RT}{M}}$
$mathrm{v_{rms}=sqrt{frac{3 imes 8.31 imes 283}{4 imes 10^{-3}}}}$
$mathrm{v_{rms}=1.76 imes :10^6: m/s}$
因此,氦气在10°C下的均方根为$mathrm{1.76倍:10^{6}:m/s}$
