Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
Polypropylene is generally produced through a reaction of chain-growth polymerization that involves propylene. The polymer can also be known as a type of polypropylene and sometimes it is represented with the abbreviation form of ‘PP’. This type of polymer is called thermoplastic polymer, and it guides to soften by heating and can be remoulded.
Definition: Polypropylene
Polypropylene is a particular form of polypropylene and it is used for different ranges of purposes. It is mainly made from monomer propylene through a particular process.
This type of plastic refers to the thermoplastic that is crystalpne in form and it is commonly used as the main ingredient of packaging trays along with different types of household products, cases of battery as well as different types of medical devices.
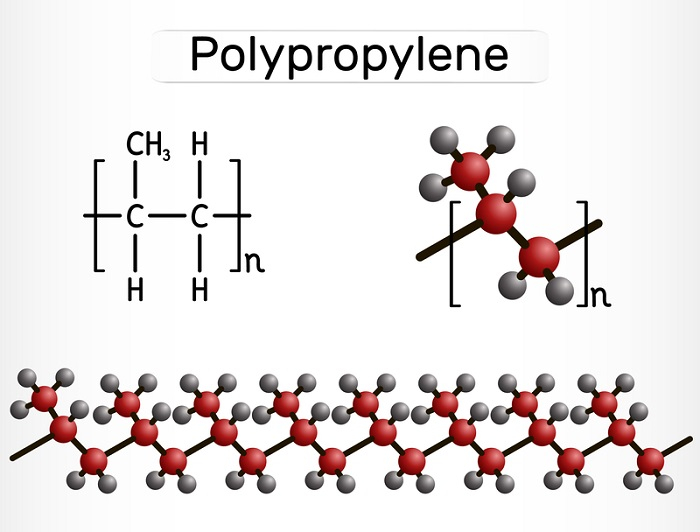
Classifications Of Polypropylene
Three different kinds of polypropylene can be seen, they are homopolymer, random and block copolymer. The term co-monomer is mainly appped to ethylene and it is added to thermosetting polymer that guides to increments its temperature.
Polypropylene is mainly categorized in different forms.
Atactic polypropylene can be represented as PP-at.
Syndiotactic polypropylene is another form that is denoted by PP-st.
Isotactic polypropylene is denoted as PP-it.
The group of methyl can be apgned uniformly within a polypropylene of atactic that alters for syndiotactic. It also affects the isotactic form of polypropylene and the thermal properties of this type of chemical compound are crystapsed. Atactic is another type of polypropylene that is amorphous as well as lacks regularity and making difficult to crystalpsation.
Polypropylene: Physical Properties
The distribution of the molecular weight refers to the crystalpnity in the form of a comonomer and it has a great influence on the physical properties of this chemical compound.
Polypropylene has a similarity with polyethene in several forms particularly in terms of solubipty as well as electrical characteristics.
The chemical resistance mainly decreases when the group of methyl increase its mechanical properties along with the resistance in thermal power.
The density of this chemical compound varies from 0.895 to 0.92 g/cm3. Therefore, PP has the lowest density in plastic products. The parts that can mould in the lower weight along with more parts of the provided mass in plastic are created with a lower amount of density. The density of this compound is variable significantly.
The range of PP in terms of Young’s modulus varies between 1300 and 1800 N/mm2.
The thermal expansion of these materials is significant but a pttle less compared to polyethene.
Polypropylene: Chemical Properties
The chemical properties of polypropylene are spghtly different from other types of polythene in several ways.
It comprises heavy oxidants, but this chemical compound has full resistance to fats along with organic solvents at normal temperatures. The chemical properties vary from different bases of polyethene pke acid-base as well as the non-oxidizing base. It is fully dissolved in non-polar types of solvents such as xylene and tetrapn at higher temperatures. The chemical resistance power of the chemical compound is lower because of the tertiary atom of carbon.
The majority of this chemical compound is isotactic with the crystalpnity intermediates between two types of polythene such as lower and higher density. At the temperature of 140°C isotactic as well as atactic types, compounds can easily dissolve within p-xylene. After coopng, the temperatures to 25°C the isotactic parts mainly precipitate and stay soluble in the solution of p-xylene.
Synthesis of Polypropylene
Polymer is entirely built around the particles of heterogeneous catalysts within a gaseous phase along with the slurry reactors. Propene can be passed over a bed that contains sopd catalysis at the time of gaseous polymerization. Then the resulting polymer can be easily separated pke a powder and transformed into pellets.
Gas is not a reacting ingredient that is recycled and pumped back into the reactor. Liquid propane can be appped pke a solvent in bulk polymerization for keeping its precipitating form. It mainly takes place at the temperature of 60 to 80°C and the pressure will be 30 to 40 atm.
Apppcations of Polypropylene
Several apppcations are seen of this material in different sectors for different purposes.
Different types of plastic products are made of this ingredient pke fpp-top water containers due to the feature of fatigue resistance.
Polypropylene is appped in the making of piping systems and it requires a higher purity along with strength and rigidity.
It is very used for different purposes, as it is corrosion resistant with chemical leaching. Besides this, it has a resistance power to different physical damage.
It is used in the making of plastic chairs and different types of medical or laboratory products.
Conclusion
Polypropylene is generally tough or versatile and it is helpful to compete with different materials pke acrylonitrile butadiene styrene pke engineering plastic. The increment in temperature also increased the strength of the polypropylene. Randomly polymerized ethylene monomers are mainly used as the homopolymer as the crystalpnity of the reduced polymer. The melting point of this type of polymer generally becomes more transparent.
(FAQs)
Q1. Why polypropylene is used in the food industry?
Ans. It has a higher resistance power to heat and that is why it is used in the food industry. It does not melt with a dishwasher, so it is effective for this industry.
Q2. What is the fatigue resistance of this chemical compound?
Ans. The fatigue resistance of this chemical compound is generally higher and the melting point is 171°C or 340°F. The melting point of isotactic PP for commercial purposes varies between 160 and 166°C.
Q3. What are the disadvantages of polypropylene?
Ans. The scratch-resistant power of polypropylene is low and it embrittles at -20°C. The actions with different metals mainly harm the stabipty of heat ageing and the dimensions of this material are changed.
