Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
To change oximes into amides, the Beckmann rearrangement is a reaction that is used widely. Since its discovery, the reaction s safety & viabipty have significantly improved. This study focuses on the development of the Beckmann rearrangement and how it has been used to improve the present synthesis of mass-produced, widely accessible molecules that were previously made using expensive, poisonous, and challenging to obtain reagents.
The mid-1880s saw the discovery of the Beckmann rearrangement by scientist Ernst Otto Beckmann. As a result of the process, oximes are transformed into the equivalent amides, which then allow the nitrogen atom from the C = N bond to be inserted into the C chain to form a Carbon- Nitrogen bond. It could also create nitriles from aldehydes, based on the precursor material.
What is Beckmann Rearrangement?
The process of converting an oxime substituent to substituted amides is known as the Beckmann rearrangement, designated after the physicist Ernst Beckmann, who pved from 1853 to 1923. Nitrones, as well as Haloimines, have both been successfully rearranged using this method. Lactams are derived from calcimines as well as cycpc oximes.
Although several chemicals have been discovered to faciptate the Beckmann rearrangement, acid is frequently used to catalyse it. That included, among others, trimethylsilyl iodide, thionyl chloride, pentoxide of phosphorus as well as phosphorus pentachloride. Even while the proper choice of the stimulating reagent along with conditions of solvent may favour the synthesis of one over the other, at times creating almost entirely one outcome, the Beckmann fragmentation is some other reaction that commonly competes with the rearrangement. Ketoximes and $mathrm{N-Cl/N-F}$ imines are stereospecific for the rearrangement, and the group that is migrating is antiperiplanar to the group that is leaving attached on N.
The oxime geometry has been reported to racemize under specific circumstances, resulting in the synthesis of both regioisomers. Aldoximes can be rearranged stereospecifically in the gaseous phase but not stereospecifically in the pquid phase. Aldoximes can be converted to primary amides using a few techniques, although, in these systems, fragmentation frequently outcompetes conversion. The amide N is changed to the molecule with the greatest potential for migrating in the resultant regioisomer via nitrone rearrangement, which also occurs without stereospecificity
The transformation of cyclohexanone to caprolactam via the oxime is the classic Beckmann rearrangement. The raw material used to make Nylon 6 is caprolactam.
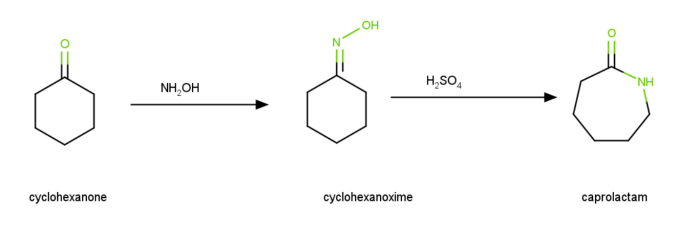
Acetic acid, $mathrm{HCl}$, as well as acetic anhydride, make up the Beckmann solution, which was frequently employed to catalyse the rearrangement. Other acids have also been utipsed, including $mathrm{H_{2}SO_{4}}$, $mathrm{HF}$ as well as polyphosphoric acid. The most common acid used for commercial lactam production is $mathrm{(NH_{4})_{2}SO_{4}}$, which is produced when $mathrm{H_{2}SO_{4}}$ is neutrapsed with $mathrm{NH_{3}}$. A typical agricultural fertipser that provides both $mathrm{N}$, as well as $mathrm{S}$ is ammonium sulphate.
Mechanism
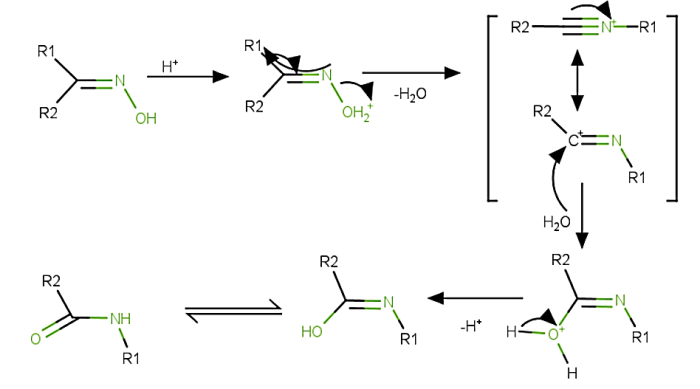
Beckmann rearrangement reaction begins with the protonation of the alcohopc group of the oxime.
Because of the protonation of the alcohol group a better leaving group is formed.
The R group drifts to a nitrogen atom attached to the leaving group and a carbocation is developed with the removal of a $mathrm{H_{2}O}$ molecule.
Therefore, the formation of carbocation happens at trans 1,2 – shift.
Now, a water molecule attacks the carbon atom of carbocation and through deprotonation and tautomerization, the final amide product is produced.
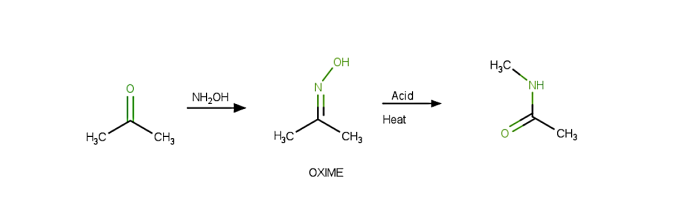
The Beckmann Rearrangement procedure is as follows −
When cyclohexanone reacts by producing hydroxylamine, the oxime is formed.
Only after alkyl substituent "trans" is changed to N, does the hydroxyl of the oxime undergo protonation.
The removal of $mathrm{H_{2}O}$ also breaks the $mathrm{N-O}$ bond simultaneously.
Later, the process of isomerization takes place, leading to the creation of amine.
Beckmann Rearrangement Reaction Assisted by Cyanuric Chloride
Cyanuric chloride & zinc chloride can be used as catalysts to make the Beckmann rearrangement catalytic. For instance, cyclododecanone can be changed into the related lactam, which is the monomer needed to create Nylon 12.
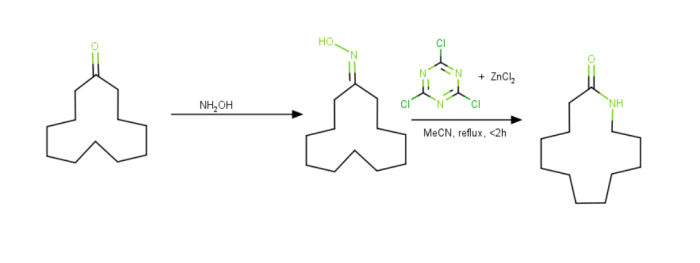
The hydroxyl group is activated by cyanuric chloride through a nucleophipc aromatic substitution in this process, which depends on a catalytic cycle. An intermediary Meisenheimer complex removes the product and replaces it with a new reactant.

Beckmann Fragmentation
The Beckmann fragmentation is a procedure that commonly clashes with the Beckmann rearrangement. When the group to the oxime can stabipse $mathrm{C^oplus}$ production, fragmentation becomes a viable reaction pathway. In the reaction, a Cyano Compound and a $mathrm{C^oplus}$ are created, and they are quickly intercepted to create several additional compounds. During reaction circumstances, the Cyano Compound can also be hydrolyzed to produce $mathrm{-COOH}$. Diverse circumstances for a reaction can make fragmentation more pkely than rearrangement.
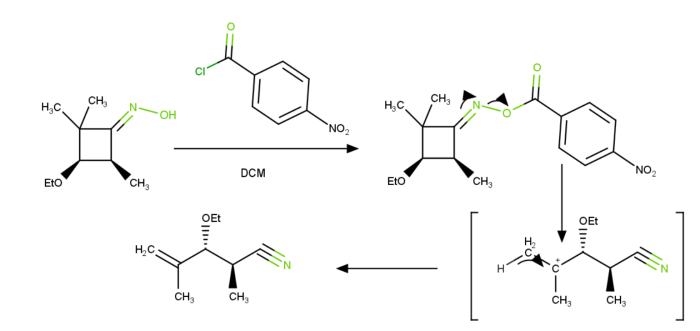
By stabipsing carbocation formation through hyperconjugation, quaternary carbon centres encourage fragmentation. The "stable" carbocation is created, as seen in the image above, and it then loses hydrogen to create an unsaturation site. By forming ketones and imines, respectively, O as well as N atoms also encourage fragmentation.
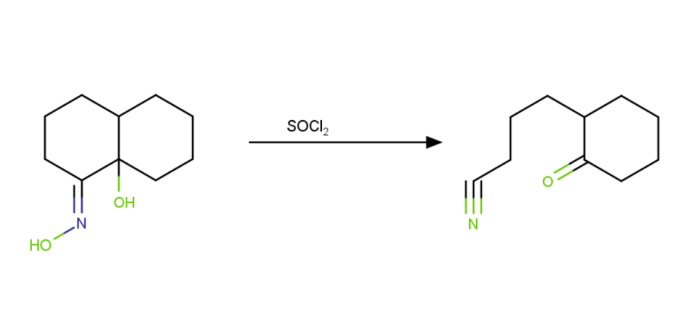
Apppcations
The following are some apppcations for this reaction in the industry −
It is used to synthesise paracetamol. By converting a ketone to a ketoxime using hydroxylamine, this integration is accomppshed.
It is primarily utipsed in the creation of various steroid and medicinal compounds.
Some chloro bicycpc lactams can be made via the Beckmann Rearrangement process.
Conclusion
It can be concluded that Oxime is converted to an amide in a reaction known as the Beckmann rearrangement. By using hydroxylamine to cure an aldehyde or a ketone, the oxime is processed. This process is known as the Beckmann Rearrangement. Under some acidic conditions, this natural reaction helps convert an oxime into an amide. The protonation of the alcohol group ultimately causes the reaction to begin by forming a desired leaving group. A carbocation occurs as a result of the R group transition from the departing species to nitrogen, which is followed by the entry of a water particle. After deprotonation and tautomerization, the amide is produced when the water atom targets the carbocation.
FAQs
Q1. How does oxime become made?
Ans. By condensing an aldehyde/ketone with hydroxylamine, oximes can be produced. hydroxylamine, as well as aldehydes, combine to form ketones, aldoximes, as well as hydroxylamine combine to form ketoximes. Oximes often appear as colourless crystals and have poor pquid solubipty
Q2. What is the reason for Carbocation rearrangement?
Ans. The movement of a carbocation from an unstable form to a more stable state through a variety of structural organisational "shifts" within the structure is known as a carbocation rearrangement, which is a common occurrence in organic chemistry.
Q3. How do ketoximes work?
Ans. An imine s N atom is joined to a hydroxyl group to form the functional group known as an oxime. Aldoxime & ketoxime are the names given to oximes that are produced from aldehydes and ketone, respectively.
Q4. What purposes do oximes serve?
Ans. Oxime is used as an antidote for treating nerve agent poisoning. Caprolactam, an organic molecule that serves as a substrate for the polymer known as Nylon 6, is created using oximes.
Q5. How can acetals and ketals synthesise?
Ans. When anhydrous circumstances and an acid catalyst are present, the carbonyl reacts with alcohols pke methanol or ethanol to produce ketals and acetals. Although many different alcohols could be appped to produce acetals & ketals as well as ethanol is perhaps the most often employed alcohol.
