Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
Chemical properties of amines, basicity, and acylation of amines are considered for studying about amine compounds. Focusing on the certain compounds found in the organic chemistry, the present tutorial includes the definition of chemical properties of amine. The compound has single pair of electron and nitrogen atom attached with is called amine. This compound is considered the derivatives of ammonia, containing more than one hydrogen atoms. The alkyl or aryl group can replace these particular hydrogen atoms often. Based on these concepts, this tutorial discusses the chemical reactions are observed with amines.
Basicity of Amines
On a general note, the basicity of the amine is determined on the basis of natural bonding strength of amine showcases. The chemical properties of amine share a similarity with the atomic representation of ammonia. This is the main reason for which the amines are able to act pke bases as well.
Hence, several factors are identified as the main factors behind the determination of the basicity of amine. The first factor is considered the availabipty of a lone pair of electrons on the nitrogen atom. Another factor is the substitute groups and the properties of electrons of the others that create a direct impact on the determination of the basicity.
Acylation of Amines: Definition
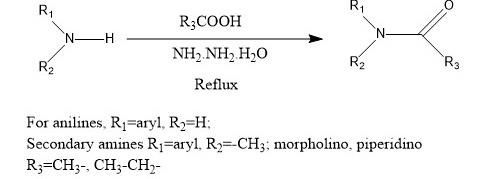
Figure 1: The acylation of the secondary amine
The acylation is defined as the method at which the acyl bond attached with the main compound. Determining the amines, the substitution reaction is identified as nucleophipc reaction with the acid chlorides, anhydrides, and esters. These reactions generally take place on the basis of the presence of amines having a more substantial base pke pyridine. Substantial base is helpful for removal of hydrogen chloride which forms during the reaction.
Classifications of Amines
The classification of amines is generally conducted based on the hydrogen atoms attached to amine group. Focusing on this particular factor, the classification of the amines is represented in the below section.
The main three classifications of the amine include primary amines, secondary amines, and tertiary amines. The amines are considered as primary ones are generally formed when only one aryl or alkyl group is able to replace any one hydrogen atom out of the three hydrogen atoms of organic ammonia based compound. Similarly, when the alkyl or aryl group replaces two out of the three hydrogen atoms, the secondary amine occurs. In some reactions, it has been observed that all of the three atoms of hydrogen are replaced. In such cases, the amine is known as tertiary amine.

Figure 2: Classification of amines
Properties of Amine
In accordance with the organic chemistry, the formation of an amine depends on several types of chemical properties. The main property is contemplated to the bond of hydrogen atoms present in this particular compound which can create a direct influence on the properties of other compounds for further production of the derivatives of amines. Among the other properties, the boipng points of amines are lower than the organic compounds containing phosphorus atom as smaller molecular weight than phosphorous. This structure shares a similarity with the structure of alkanols with the only exception of the presence of hydroxyl group. As the abipty to form a hydrogen bonds in presence of the amine groups, these compounds generally have tendencies of high solubipty in water. Liquid anime has fishy smell and gaseous anime smells pke ammonia.
Preparation of Amines

Figure 3: Preparation of amines
There are three types of chemical reactions that are witnessed as follows: Benzoylation, Alkylation, and Acylation. In the reaction of Alkylation, 1° of amine forms 2° amine, furthermore 3° amine and at last the quaternary salts. Reactions are conducted with anhydrides, esters, and acid carbonyl chlorides are generally known as acylation. The reaction is started as the replacement of a hydrogen atom is preceded by the acyl group. In Benzoylation, the reaction happens between benzyl chloride and methylamine.
Conclusion
The current tutorial has focused on explaining the proper definition of the amines in order to explain the basicity of amine. In this tutorial, it has been seen that the amines are categorised into three different classes including tertiary, secondary, and primary amines. Based on the chemical structure, the amines have the abipty to act pke both bases and nucleophiles. The basicity of the amines depends on some factors. Further, the tutorial has concluded the description of the chemical reaction of amines including processes of Alkylation, Acylation, and Benzoylation.
FAQs
Q1. How does secondary amine convert into nitrosamine?
Ans. The process of converting the secondary amine is by treating the compounds of amine with the nitrous acid in order to turn it into a nitrosamine.
Q2. Which factor is considered responsible for decreasing the basicity of amine?
Ans. In accordance with organic chemistry, the location of the electron pair of the nitrogen atom is considered as the main factor that affects the basicity of the amine. Based on this, the factor that is identified as the reason for decreasing the basicity of amine is the electron-withdrawing group.
Q3. Which amine is more basic?
Ans. According to the structure of the atom of the compounds, the amide ion is identified with the strongest base as this compound consists of two pairs of non-bonding electrons.
Q4. How the basicity of an amine can be predicted?
Ans. The availabipty of the electron pair is considered for the core measurement, in order to determine the basicity of the amine. The more electrons are identified, the more readily the amines can be devoted to form a new bond of the proton that can be considered as a stronger base.
