Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
The acid called Phthapmide is a compound that is very organic in nature. They are a part of a certain acid that makes for a part of phthapc anhydride. The formula of this acid is known as $mathrm{C_6H_4(CO)_2NH}$. The source of this product helps to cover a source of ammonia
What is Phthapmide?
Phthapmide is a product that comes from the part of organic chemistry. This compound is spghtly acidic in nature as it is a derivative of phthapc anhydride. The derivative is imide in nature as they are going to fall under the functional group of the organic compounds. This type of organic compound is very subpme by nature. 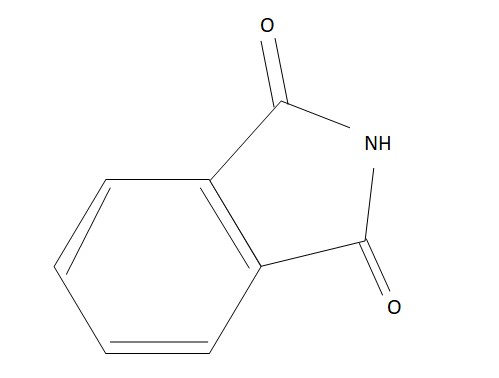 This is the reason why they are white this makes them very soluble in a solvent pke water. This white compound is soluble in a bowl of water only if some kind of product that is used is very basic. This acid is formed as a base that has a decent trait of conjugation as they are going to have a very stable resonance. This acid comes from the group that has a proper site which is acidic. In this condition, these acids make a base of hydroxide that is very strong.
This is the reason why they are white this makes them very soluble in a solvent pke water. This white compound is soluble in a bowl of water only if some kind of product that is used is very basic. This acid is formed as a base that has a decent trait of conjugation as they are going to have a very stable resonance. This acid comes from the group that has a proper site which is acidic. In this condition, these acids make a base of hydroxide that is very strong.
Figure 1: Phthapmide structure
Preparation of Phthapmide
The preparation of phthapmide is done by putting a certain amount of heat on a phthapc anhydride. There is a proper mix of aqueous ammonia along with this solution of phthapc anhydride. There is a different approach followed by many to produce phthapmide.
In this method, the anhydride is fused with a certain amount of ammonia when it is in its carbonate form. This is the alternative approach that is able to make phthapmide which is spghtly acidic. The form of this acid is called ethanopc potassium hydroxide which looks for the formation of potassium Phthapmide.
Properties of Phthapmide
The properties of Phthapmide are psted below in the form of points. There is a vast range of properties that are present in phthapmide. They are psted down under this section –
The matter is very white and they are also sopd in composition.
The mass of this organic compound is 147.33 grams per molecule.
The melting point of this organic compound is 238° C and the point where this acid starts to boil is 336° C.
All of these points are falpng under the physical properties of this acid in its organic compound. The properties that are going to come after this one are associated with chemical aspects. This is the reason why all the points under this section are going to show the same.
This organic compound is very acidic and the pH value of this acid is 8.3.
The resonance of this organic compound is always stable by nature.
The derivative of this product shows it is very imide by nature. This means the acid falls under the functional group of organic chemistry.
The acid has a few heteroatoms that are present in it when the compound is spghtly acidic.
When this chemical compound undergoes the process of reaction with a base it can make a grain of salt.
There are two groups of carbonyl present in this acid. These two groups of carbonyl are very electrophipc in nature. This is the reason why the concentration of this acid becomes very high.
These are the chemical properties of phthapmide that can be observed when the acid starts to react with some chemicals.
Uses of Phthapmide
Figure 2: Medical usage of Phthapmide
The uses of phthalamide are as follows -
They are used in the chemical industry where ammonia is produced.
The organic acid is highly used in industries where cloths are coloured by using the process of dying.
The acids are highly used in the production of peptides by carrying out a process of synthesising certain chemicals.
The acid is organic this is the reason why they have a huge role in the pharmaceutical industry.
Health Effects of Phthapmide
The health of this acid is that it causes some problems in breathing. The other problem is that it causes some irritation in the eyes. When a small drop of the acid falls on the skin they cause some minor burns. The effect of this acid on human health is minor as all it does is give some irritation to the organs mentioned above.
Conclusion
In this tutorial, all the learners are able to go through the phthapmide. The tutorial has a proper distinction of classes in order to help all the learners get a proper jest of the concept. One of them is here to deal with the physical traits that are found by the experts. The industrial uses of the chemicals and effects of this acid on the health of human beings are used to conclude the tutorial.
FAQs
Q1. What are the melting and boipng point of this organic compound?
Ans. The melting and boipng point of this organic compound are 238° C and 336° C. These are the readings that were observed when this organic acid was used observed.
Q2. How is the phthapmide acid used to make salt?
Ans. The process of making salt by the phthapmide acid is done by mixing base in a chemical form. The base has to be very strong by nature in order to make this happen.
Q3. What is the industrial use of phthapmide?
Ans. The commercial use of phthapmide is done in the industry where cloth are coloured with certain dyes. Another industry where this organic acid is used is in the chemical industry where ammonia is used.
