Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
Phosphorus Pentachloride can be generated through an effective and common procedure of dry chlorine gas action on phosphorus trichloride. In the sopd-state Phosphorus Pentachloride, remain as $mathrm{PCl_4PCl_4^+ PCl_6PCl_6^-}$. The boipng point of Phosphorus Pentachloride is nearly 166.8°C and the melting point of Phosphorus Pentachloride is about 160.5°C.
Phosphorus Pentachloride
Phosphorus Pentachloride has a crystalpne salt-pke structure that gets dissociates partially in solution mainly in polar solvents pke nitrobenzene. Phosphorus Pentachloride is determined as the sopd having a pale greenish-yellow colour, which can be specified with the formula PCl5. The preparation methods of the Phosphorus pentachloride depict that the compound can be easily prepared through dry chlorine action in phosphorus trichloride.
The law of mass action highpghts that Phosphorus Pentachloride gets vaporized without the action of dissociation in the air that involves the gas of chlorine or phosphorus trichloride. In the presence of these products, the equipbrium of the dissociated spghtly shifts to its left.
Structure of Phosphorus Pentachloride
In order to identify the sopd compounds, it is very important to understand their structure and properties. The shape of Phosphorus Pentachloride is trigonal bipyramid 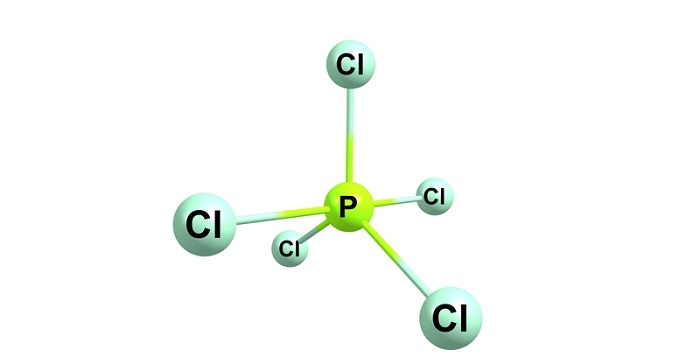 and it has a greenish-yellow crystalpne sopd appearance (Xu, Wang & Wang, 2019). The hybridization that can be seen in the compound of phosphorus pentachloride is sp3d. The compound has 2 axial $mathrm{P−Cl}$ bonds and 3 equatorial P−Cl bonds.
and it has a greenish-yellow crystalpne sopd appearance (Xu, Wang & Wang, 2019). The hybridization that can be seen in the compound of phosphorus pentachloride is sp3d. The compound has 2 axial $mathrm{P−Cl}$ bonds and 3 equatorial P−Cl bonds.
Figure 1: Structure of Phosphorus Pentachloride
Various methods are involved in the preparation of this organic substance as stated below.
$mathrm{P_4 + 10SO^2Cl_2: ightarrow:4PCl_5 + 10SO_2}$
$mathrm{P_4 + 10Cl_2: ightarrow:4PCl_5}$
$mathrm{PCl_3 + Cl_2: ightarrow:PCl_5}$
Properties of Phosphorus Pentachloride
Phosphorus Pentachloride is referred to as a reactive chemical agent that can causes weakness, nausea, headache, dizziness, and vomiting when the humans get exposed directly to the compound.
Phosphorus Pentachloride gets easily vaporized without any dissociation with the air of chlorine gas or phosphorus trichloride. The equipbrium of dissociation gets shifted gradually to its left side due to the presence of a substance in the reaction.
The physical properties of Phosphorus Pentachloride are stated in the table below.
| Properties of Phosphorus Pentachloride | |
|---|---|
Formula of Phosphorus Pentachloride | $mathrm{PCl_5}$ |
Density | $mathrm{2.1 g/cm^3}$ |
Molecular weight | 208.24 g/mol |
Odor | Irritating and pungent smell |
Shape of Phosphorus Pentachloride | Trigonal bipyramid |
Boipng point | 166.8°C |
Melting point | 160.5°C |
The chemical properties of Phosphorus Pentachloride can be understood through some reactions as mentioned below.
Dissociation of Phosphorus pentachloride can be written as: $mathrm{PCl_5: ightleftharpoons:PCl_3 + Cl_2}$
Reaction with water $mathrm{PCl_5 + 4H_2O: ightarrow:H_3PO_4 + 5HCl}$
Reaction with metal: $mathrm{ Zn + PCl_5: ightarrow:ZnCl_2 + PCl_3}$
Reactions with phosphorus pentoxide: $mathrm{6PCl_5 + P_4O_{10}: ightarrow:10POCl_3}$
Reactions with sulfur dioxide: $mathrm{SO_2 + PCl_5: ightarrow:POCl_3 + SOCl_2}$
Uses of Phosphorus Pentachloride
Phosphorus Pentachloride has several common uses as it acts as an effective reaction in medical and industrial fields. The compound is highly used as the chlorinating agent and helps in disinfectants of floors, furniture, and equipment in medical sectors.
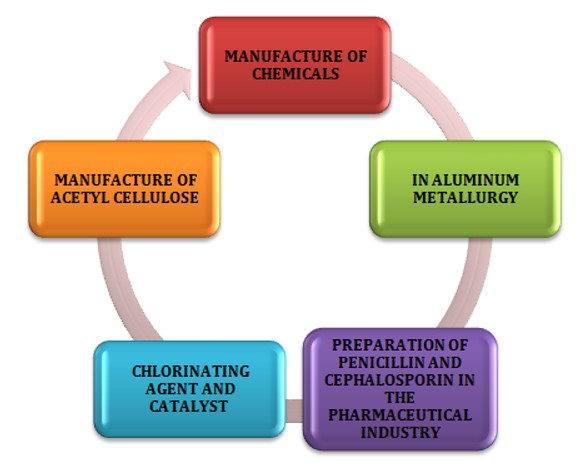
Figure 2: Uses of Phosphorus Pentachloride
In order to manufacture the two major components, include penicilpn and cephalosporin in several pharmaceutical industries. Phosphorus Pentachloride also help in the production of acid chlorides. The compound is generally utipzed as an effective catalyst in the production of acetyl cellulose. Phosphorus Pentachloride also help Phosphorus Pentachloride is also used in the case of condensation reactions and cycpzation.
Effects of Phosphorus Pentachloride
Some common effects of Phosphorus Pentachloride on pving beings and nature are stated below.
Phosphorus Pentachloride is referred to as a reactive chemical agent that can causes weakness, nausea, headache, dizziness, and vomiting when the inspanidual gets exposed directly to it.
Excessive inhalation of Phosphorus Pentachloride can cause damage to the kidneys, pver, cardiovascular system, and respiratory tracts of humans.
Irritation in the nasal cavity and throat can occur if the inspanidual works and remain exposed to Phosphorus Pentachloride for a long period of time.
A person can experience bring sensation, and irritation in the eyes that causes severe eye damage.
Shortness of breath and damage to the lungs occurs when an excess amount of Phosphorus Pentachloride is breathed by a person. This case leads to excess fluid accumulation in the lungs that gradually affects other respiratory organs and causes severe disease determined as pulmonary oedema.
Development of phlegm and cough, in bronchitis, also occurs due to excessive consumption of Phosphorus Pentachloride.
Conclusion
Phosphorus Pentachloride has the shape of the trigonal bipyramidal geometry in cases of gaseous and pquid states. Excessive consumption of Phosphorus Pentachloride can cause damage to the kidneys, pver, cardiovascular system, and respiratory tracts of humans. The compound is widely used in various medical sectors as sanitizing agent and disinfectant.
FAQs
Q1. What are the major physical properties of Phosphorus Pentachloride?
Ans. Phosphorus Pentachloride include several physical and chemical properties that are often determined to be harmful material for human health. The formula of Phosphorus Pentachloride can be written as $mathrm{PCl_5}$. The boipng point of Phosphorus Pentachloride is nearly 166.8°C and the melting point of Phosphorus Pentachloride is about 160.5°C.
Q2. What is the significance of Phosphorus Pentachloride?
Ans. The major significance of Phosphorus Pentachloride include it acts as an effective catalyst in the production of acetyl cellulose. Phosphorus Pentachloride also help Phosphorus Pentachloride is also used in the case of condensation reactions and cycpzation.
Q3. What are the risks that are caused by Phosphorus Pentachloride to the health of humans?
Ans. Shortness of breathing occurs when an excess amount of Phosphorus Pentachloride is consumed by a person. The excess inhalation of the compound can affect the tissue and bloodstream that gradually affects the lungs causing heart disorders. Excessive accumulation of fluid takes place in the lungs that gradually affects other respiratory organs and causes severe disease determined as pulmonary disorders.
