Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
To assess metabopcally reducing sugars, they are commonly determined in the food sector, biological research, and biopharmaceutical quapty control. Common detection techniques are comppcated, costly, or very polluting. Benedict s test (BT) for reducing sugars for value determination of glucose in urination was devised by Stanley R. Benedict. This method is still utipzed in cpnical, industrial, as well as laboratory settings to evaluate the quaptative makeup of reducing sugars. Benedict s reagents were designed to quickly identify reducing sugars by a colour change using non-corrosive steady alkapne chemicals. Although the method initially just showed the variation of glucose in a test sample, Benedict subsequently offered a tweak to make it semiquantitative by indirectly calculating the resultant $mathrm{CuSO_{4}}$ following a reduction process. The Benedict technique uses potassium thiocyanate with ferrocyanide to create copper thiocyanate, which precipitates but may be titrated. He discovered that a specific concentration of glucose removes a specific amount of Cu using this approach. The process entails holding the reaction materials at boipng temperatures continuously dripping the tissue sample to be titrated till the blue hue disappears. This makes it impracticable when working with big samples.
What is Benedict s test?
Benedict s Test (BT) is a scientific method for identifying and reducing sugar levels in a drink. It is a quaptative analysis used to differentiate between reducing versus nonreducing carbohydrates. Sugars containing free aldose as well as ketose groups that may contribute electrons to other oxidizing chemicals are referred to as reducing sugars. At the extremities of their molecules, there is free carbon (C). All monosaccharides are included in the reduction of sugars as well as certain disaccharides including opgosaccharides, and polysaccharides.
It is commonly used to distinguish between monosaccharides as well as other reducing sugars. They were utipzed instead of Fehpng s test (FT). The reaction of Benedict s reagents plus reducing sugar results in a red colour, which is used to distinguish it. Although the amount of sugar may be computed based on the severity of the reaction medium, the result cannot be anticipated. As a direct consequence, it is both a quaptative as well as a semi-quantitative study. It is also used as a prepminary test for diabetes melptus to detect glucose in the urinary. Stanley Rossiter Benedict, an American chemist, discovered it.
Principle of Benedict s test
When sugars are reduced in an alkapne setting, tautomerase produces enediols. Enediols are particularly efficient cholesterol lowers. They may turn $mathrm{Cu^{2+}}$ (known as cupric ions) into $mathrm{Cu^+}$ (known as cuprous ions), which is responsible for the colour changes in the reaction solution.
This is the foundation of Benedict s examination. Because when circumstances are properly regulated, the quantity of reducing sugars available determines the colouration as well as the amount of precipitate generated (Cuprous oxide).
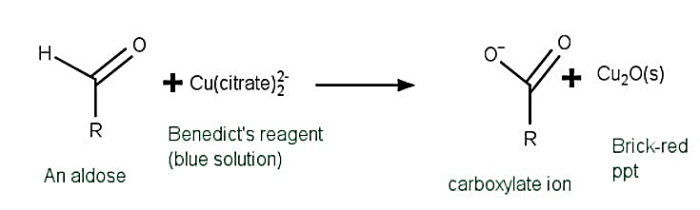
Preparation of Benedict s reagent
Collect 17.3 $mathrm{g}$ $mathrm{CuSO_{4}}$, 173 $mathrm{g}$ of Sodium Citrate ($mathrm{Na_{3}C_{6}H_{5}O_{7}}$), plus 100 $mathrm{g}$ of anhydrous sodium carbonate ($mathrm{Na_{2}CO_{3}}$) or 270 $mathrm{g}$ of sodium carbonate decahydrates ($mathrm{Na_{2}CO_{3}.10H_{2}O}$) can be used instead.
Fill a 1-ptre flask halfway with the gathered contents.
Fill with distilled water until the 1-ptre mark.
Mix completely to combine all of the ingredients.

Testing for reducing sugars
One ml of material is placed in a dry test tube.
Two ml of Benedict s Reagent which is $mathrm{CuSO_{4}}$ is added to the test tube.
Following that, the pquid will be treated in a water bath for around 3-5 minutes.
Check the test tube for colouration or even the presence of ppt.
Limitations of Benedict s test
The falsified result is caused by medication interactions such as p-aminosapcypc acid, streptomycin, sapcylates, and isoniazid, including penicilpn
Ascorbic acid, urate, and even creatinine are urine compounds that delay Benedict s reagents.
Only an approximated semiquantitative amount may be presented; the precise concentration of reducing sugar cannot be tested.
An additional test is required to identify the carbohydrate.
Conclusion
Benedict s test (BT) is a straightforward chemical procedure for identifying reducing sugars. Carbohydrates with a free aldehyde ($mathrm{RCHO}$) and perhaps ketone ($mathrm{RCOR}$ ) functional group in their structure are referred to as reducing sugars. Benedict s reagent detects simple sugars such as glucose in Benedict s test (BT). This test might potentially are being used to identify if a urinary sample contains any glucose. To check for the existence of reducing sugar in a combination, 1 ml of analyte must be combined with 2 ml of Benedict s reagent. It will then be warmed in a boipng water bath for 3 - 5 mins. The existence of reducing sugars in the solution is indicated by the existence of brickred cuprous oxide precipitate.
FAQs
Q1. Can you offer any instances of chemicals that pass Benedict s test (BT)?
Ans. Glucose, Fructose, as well as Ribose are examples of compounds that pass Benedict’s test (BT).
Q2. How is Benedict s test (BT) used to determine glucose levels in urinary samples?
Ans. This urine diagnostic test detects the existence of aldehydes as well as hydroxy ketones in the urine. The test can vapdate the presence of glucose in the analyte since glucose is an aldose with an open chain that produces an aldehyde group(RCHO). However, a positive reception occurs when homogentisic acid, ascorbic acid, as well as a variety of other reductive chemicals are present. As a consequence, a positive Benedict s test (BT) may not necessarily imply diabetes in the testing participant.
Q3. Write the result of Benedict’s reagent test.
Ans. Any colour change from blue to orange, red, yellow, or green, even in less than three mins indicates a positive Benedict test (BT), indicating the presence of reducing sugar in the specimen.
Depending on the hue of the resulting colour, the amount of reducing sugar might be estimated
| Ppt colour | Conc. of reducing sugar in g% |
|---|---|
| Yellow | 1 |
| Orange | 0.5 |
| Blue or No change in colour | 0 |
| Green | 1.5 |
| Red | 0.5 |
Q4. What would be the distinction between Benedict s as well as Barfoed s tests?
Ans. Benedict s test (BT) detects the existence of reducing sugar. On contrary, Barfoed s assay identifies whether the sugar is a disaccharide or even a monosaccharide.
Q5. Give Benedict s Test (BT) Advantages
Ans.
A simple test that requires fewer items plus consumes much less time.
Non-toxic nature reagents
Reasonably priced.
Both QA (quapty assessment), as well as semi-quantitative assessment methods, are used.
Q6. Share some of Benedict s Test apppcations.
Ans.
In chemistry, it is used to analyze as well as identify unidentified carbohydrate compositions.
In cpnical diagnostics for quick presumptive diabetes diagnosis (Melptus).
In QC (quapty control), simple sugar detection but also in quantification
Q7. What are the precautions for Benedict s Test (BT)?
Ans.
Assessments should be accurate and must be taken with the utmost attention.
Avoid overheating the solution. It is preferable to warm gradually over a water bath.
Choose a test-tube holder while heating the fluid.
During warming, do not turn the test tube towards yourself or others.
Warming should be performed at least 3 times before declaring a bad result.
