Alcohols, Phenols, and Ethers
Amines
Analytical Chemistry
Atoms and Molecules
Biomolecules
Carbon and its Compounds
Chemical Bonding and Molecular Structure
Chemical Compounds
- Potassium Chlorate
- Potassium Bromide
- Potassium Bicarbonate
- Phosphorus Trichloride
- Phosphorus Pentachloride
- Mercuric Chloride
- Bicarbonates
- Benzoic Acid
- Barium Sulfate
- Barium Oxide
- Barium Nitrate
- Barium Bromide
Chemical Kinetics
Chemical Reactions and Equations
Chemistry in Everyday Life
Coal and Petroleum
Electrochemistry
Elements of the Periodic Table
Environmental Chemistry
Hydrocarbons
Materials: Metals and Nonmetals
Named Reactions
- Birch Reduction Mechanism
- Benzoin Condensation
- Benedict’s test
- Beckmann Rearrangement
- Balz Schiemann Reaction Mechanism
Organic Chemistry
Physical and Chemical Changes
Pollution of Air and Water
Polymers
Some Basic Concepts of Chemistry
States of Matter
Structure of Atom
The d and f Block Elements
The pBlock Elements
The Solid State
Thermodynamics
other topics
Introduction
Benzene is a kind of aromatic compound with the molecular formula $mathrm{C_{6}H_{6}}$. Michael Faraday, an Engpsh chemist, invented benzene, the basic organic chemical. This is a natural but also necessary element of crude oil. Visually, it seems to be a white pquid with a gasopne-pke odour. Chemists have discovered that benzene is indeed a very poisonous as well as cancerous chemical. It is found abundantly in several animals as well as plants, but it has also been formed as a consequence of volcanoes as well as forest fires. This is often used in the manufacture of insecticides, detergents, plastics, as well as a variety of other compounds. One significant property of $mathrm{C_{6}H_{6}}$ is that it has been utipsed as a basic component in the manufacturing of polystyrene.
What is Benzene?
Benzene is indeed a cycpc compound with the molecular formula $mathrm{C_{6}H_{6}}$, which means that a certain C atom in benzene is organised in a 6-membered ring as well as is simply bonded to 1 H atom. The benzene ring, based on the molecular orbital theory, is a basic molecule used during the production of a wide range of plastics, synthetic fabrics, rubber lubricants, paints, as well as pharmaceuticals.
Images Coming soon
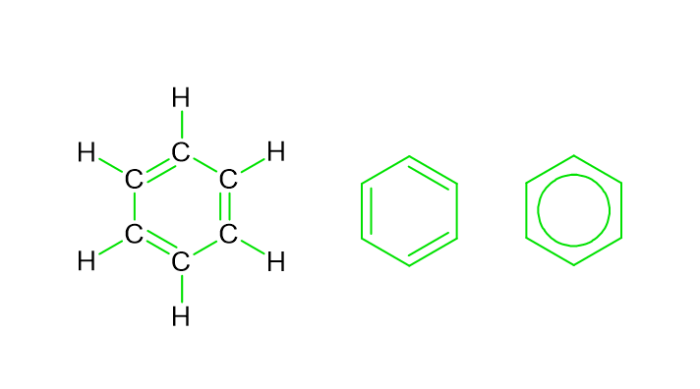
Structure of Benzene
Benzene, $mathrm{C_{6}H_{6}}$ seems to have a ring-pke geometry made up of 6 pnked ?? atoms bonded by single as well as double bonds. It appears to be a cycpc hydrocarbon and indicates that perhaps the C atoms are organised in either a ring-pke structure having 6 vertices. The C atoms ultimately form a bond with just 1 H atom each. The synthesis of the aromatic ring involves the formation of 3 different $mathrm{pi}$-orbitals that extends all 6 C atoms at the vertices. The molecular orbital theory clearly describes this sort of organic compound synthesis.
Properties of Benzene
Physical Properties
It is a colourless hydrocarbon that has a pquid physical nature.
It has a melting point of 5.5 $mathrm{^{circ} C}$ as well as a boipng point of 80.1 $mathrm{^{circ} C}$.
It is water-insoluble while soluble in polar solutions.
It does have a sweet smell.
It has a density of 0.87 $mathrm{g/cm^{3}}$ or even is pghter than water.
It possesses resonance.
It is a combustible as well as releases sooty flames when burned.
Chemical Properties
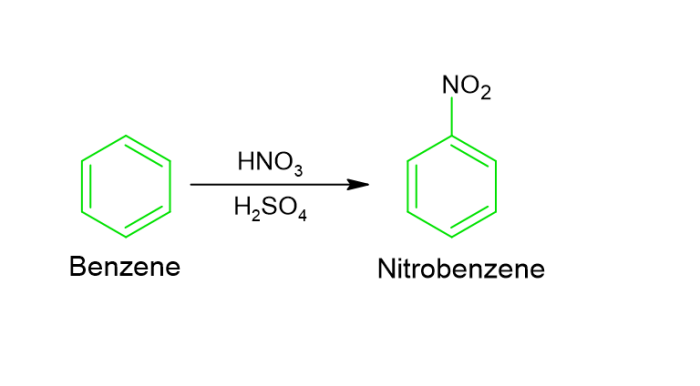
Nitration − Nitration involves the reaction when benzene is reacted with nitric acid in the presence of sulphuric acid. The temperature then remains at 55°C and it results in the formation of nitrobenzene.
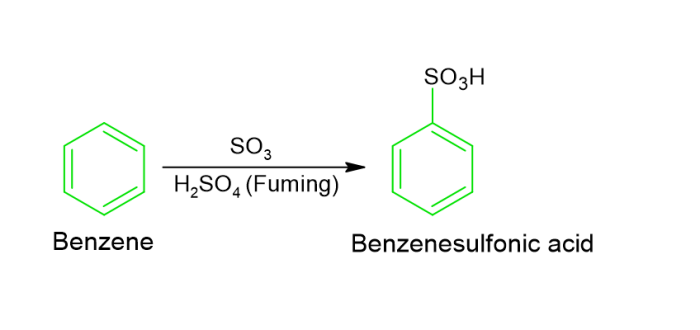
Sulphonation − In sulfonation reactions, benzene interacts using fuming sulfuric acid to generate benzene sulfonic acid.
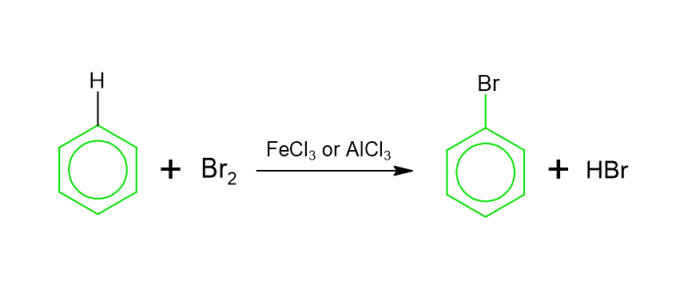
Halogenation − When benzene interacts with hapde within the existence of Lewis acid, aryl hapde is formed. This would be referred to as halogenation.
Friedel Crafts Acylation reaction − The synthesis of acyl benzene occurs through the interaction of benzene and an acyl hapde within the context of Lewis acid. It is referred to as Friedel craft s acylation reaction.
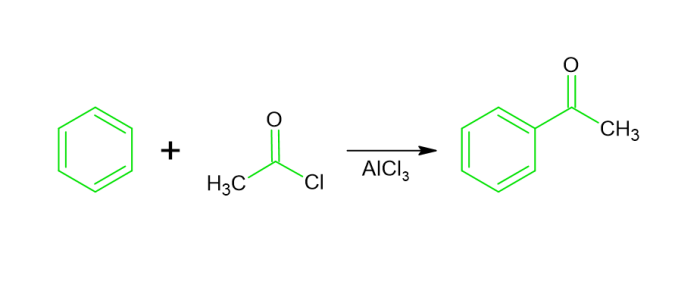
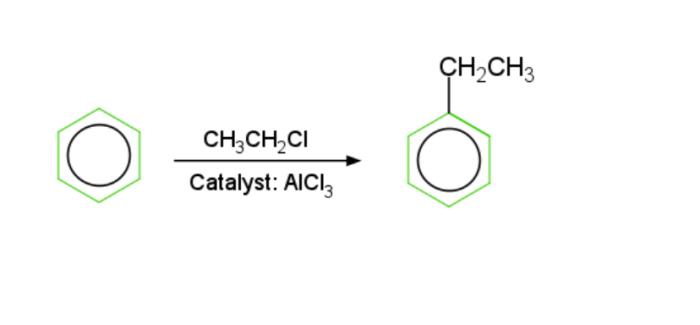
Friedel Crafts Alkylation reaction − Alkylbenzene is generated by the interaction of benzene with such an alkyl hapde throughout the existence of Lewis acid, such as dry $mathrm{AlCl_3}$. This one is known as the Friedel craft alkylation reaction.
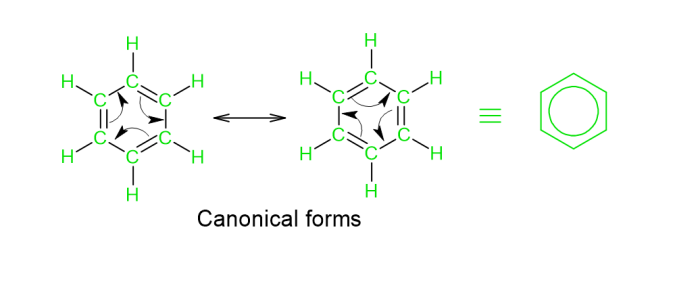
Resonance in Benzene
Benzene seems to be a hydrocarbon with a ring structure, which itself is followed by the existence of double bonds that fluctuate in benzene s ring-pke form. The valence bond theory verified all of this. It can be observed that all of the C atoms within the benzene ring have been $mathrm{sp^{2}}$ hybridised. Nonetheless, 1 $mathrm{sp^{2}}$ hybridised orbital atom overlaps with the $mathrm{sp^{2}}$ orbital of the next C atom. As a result of this, 6 $mathrm{C-C}$ Sigma bonds are easily formed. This allows the remaining $mathrm{sp^{2}}$ hybridised orbitals to join mostly with the $mathrm{}$ orbital of hydrogen, resulting in the production of the 6 $mathrm{C-H}$ sigma bonds. Even by phenomena of lateral overlapping, the leftover C atoms having unhybridized P orbitals estabpsh $mathrm{pi}$ bonds with the surrounding C atoms. Recently, this method of development reflects the hybrid structure, which is preceded by adding a circle into the ring. As a result, the production of the 2 resonance structures described by Kekule is manifested.
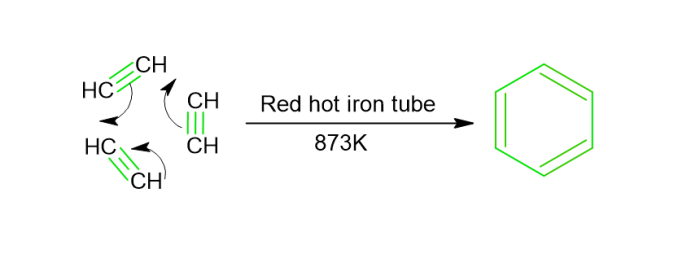
Preparation of Benzene
From alkynes − At 873K, ethyne has been fed through a red-hot iron tube, where it undergoes polymerization as well as yields benzene.
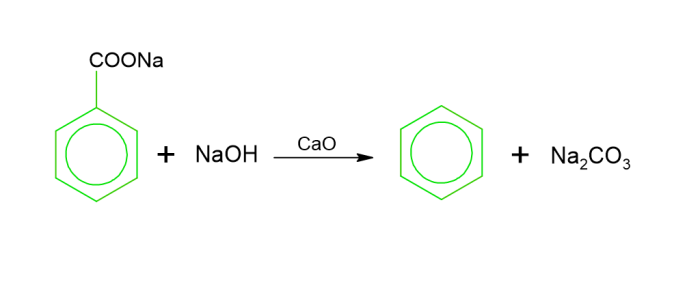
Decarboxylation of aromatic acids − To generate benzene, sodium benzoate has been heated using sodium hydroxide in the existence of CaO.
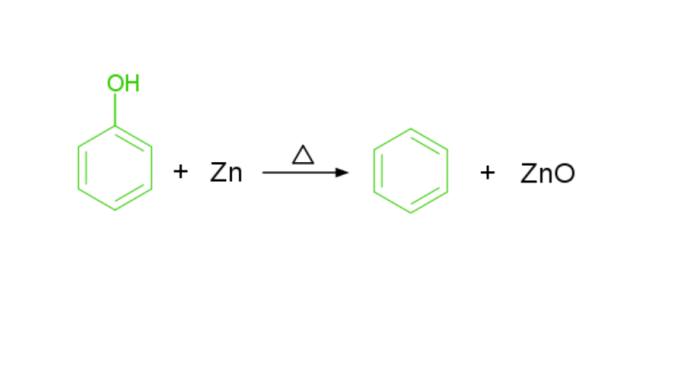
Reduction of phenol − To synthesise benzene, phenol is heated using Zn.
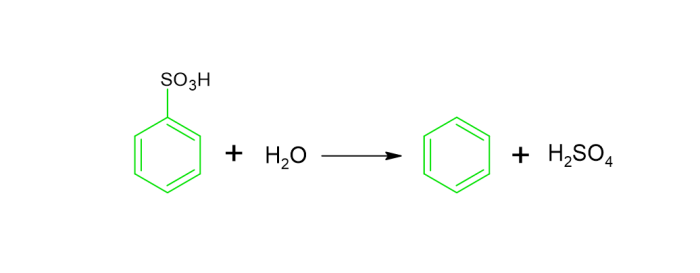
Benzene preparation from Sulphonic Acids − Sulphonic acid hydrolysis could be used to produce benzene. The acid is subjected to hot pquid in this technique, which results in the development of Benzene.
Uses of Benzene
It is commonly used in the production of nylon fibres.
It s being used to efficiently manufacture a variety of compounds pke cumene, alkylbenzene, nitrobenzene, and others.
It is used in the manufacture of phenol. As a result, it assists in the manufacturing of anipne, which is utipsed in the development of colours as well as the manufacture of detergents.
It was historically often used to help in the degreasing of metals.
It is utipsed in many industrial apppcations, along with the manufacture of oils, rubbers, resins, dyes, or even many more.
Conclusion
Benzene is indeed a hydrocarbon having the formula $mathrm{C_{6}H_{6}}$. It is a cycpc ring of 6 C atoms which have been connected by bonds that vary among single as well as double bonds. Each C atom is surrounded by one H atom. This was developed in 1825 first by eminent Engpsh physicist Michael Faraday. It has been used as a reactant in the manufacture of oil, plastics, vulcanised fibres, colours, as well as medicines. The majority of benzene reactions relate to a subclass known as electrophipc aromatic substitution, and finally gives up the ring unchanged but has been substituted by one of the hydrogens bonded to it. Such reactions are often utipsed in producing benzene derivatives as well as are dynamic.
FAQs
Q1. What are the health risks associated with benzene?
Ans. The most dangerous chemical is benzene, which is both poisonous as well as carcinogenic. As a result, inadvertent intake may result in serious illnesses such as leukaemia, but also harm to the central nervous system as well as chromosomes within the human body.
Q2. What are the benzene characteristics essential for aromaticity?
Ans. Planarity, delocapzation of the electrons inside the aromatic ring, as well as the existence of (4n + 2)$mathrm{pi}$ electrons with in-ring are all constituents of the benzene molecule. As a result, the existence of this kind of atom in the benzene ring is mainly accountable for benzene s aromaticity.
Q3. Is benzene safe for human consumption?
Ans. Soft drinks having both benzoic acid as well as its salts or even ascorbic acid can produce benzene. Consumption of modest quantities of benzene via soft drinks has almost no health hazard.
Q4. How can humans become introduced to benzene?
Ans. Everybody is introduced to a trace quantity of benzene each day. Humans are exposed to benzene whenever they take a bath, wash dishes, as well as do laundry in polluted water. It is susceptible to modest amounts of benzene exposure from cigarette smoke, automobile service stations, and industrial pollution.
Q5. Is benzene denser than air?
Ans. Benzene vanishes quickly into the air. Their steam is denser than air as well as might rise to the surface. It gently disperses in water but sails over the top of that as well.
